Large Battery Adiabatic Calorimeter—Quantifying Gas Explosion Risks: Lithium-ion Battery Thermal Runaway Testing

Background – Large Battery Adiabatic Calorimeter
With the widespread application of lithium-ion batteries in crucial sectors such as electric vehicles, energy storage, consumer electronics, and aerospace, safety concerns regarding these batteries have garnered significant attention. One of the primary safety risks associated with lithium-ion batteries is thermal runaway leading to gas evolution and explosions. This phenomenon can result in fires or even explosions, posing direct threats to user safety.
When a single lithium-ion battery cell undergoes thermal runaway due to certain triggers, it sets off a series of intense chemical reactions within the battery materials, generating a substantial amount of heat and releasing flammable and toxic gases. This buildup of internal temperature and pressure can cause the battery to rupture, leading to the release of flammable gases that may ignite upon contact with air, resulting in jet flames or explosive fireballs, thereby triggering thermal runaway in neighboring cells and causing safety incidents.
The state of charge, service life, and material composition of batteries can all influence the composition of gases evolved during their operation, consequently affecting their explosion characteristics and the risk of thermal runaway. Evaluating the explosion characteristics of battery-evolved gases is crucial for assessing the safety of lithium-ion batteries, with explosion limits being a critical parameter for assessing the hazard of combustible gases.
Explosion Limit Analysis Testing
This article explores a domestic manufacturer’s 50A·h lithium-ion battery at 100% state of charge (SOC) was subjected to thermal runaway experiments using the Large Battery Adiabatic Calorimeter (BAC-420A) in an inert gas atmosphere. Subsequently, the evolved gases were collected, and gas chromatography was employed to analyze the gas composition. The results revealed a mixture of various combustible and inert gases, which were then paired according to a specified method. The Lechteillier formula was utilized to estimate the explosion limits of the gas mixture.
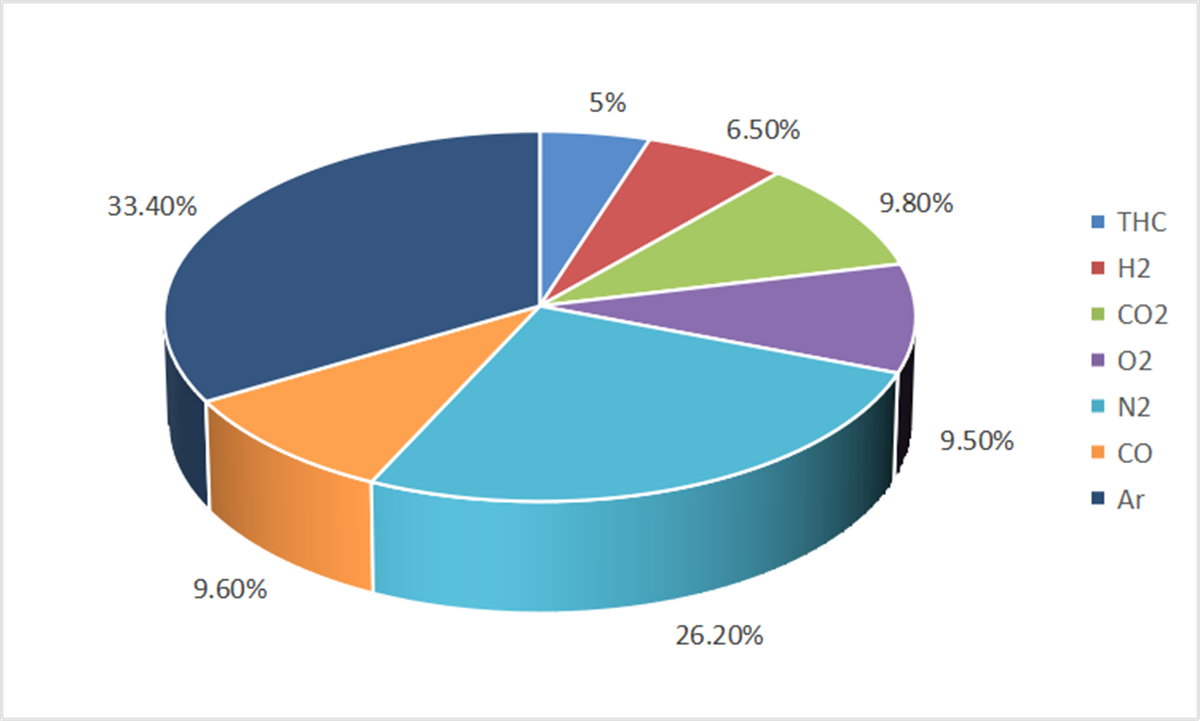
Fig. 2 Chromatographic analysis results of gas production components of a lithium-ion battery
Various combustible gases and inert gases in the mixture can be paired according to a certain method, and the explosion limit of the mixture is estimated using the Lechteillier formula [2]:
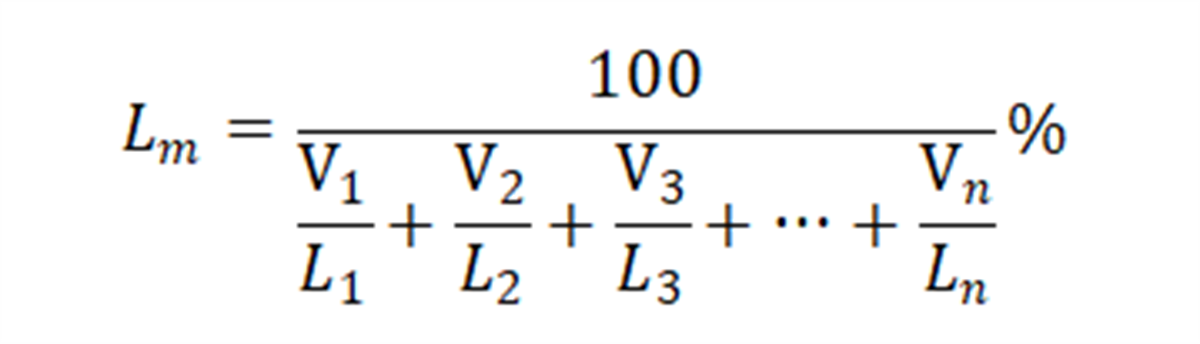
The formula calculates the explosion limit (Lm) of the gas mixture based on the explosion limits (L1, L2, …, Ln) and respective volumes (V1, V2, …, Vn) of its components. The calculated lower flammable limit (LFL) for the gas evolved from the battery was found to be 33.02%.
Next we verify the above calculations experimentally. In this case, the explosion limit tester (HWP21-30S) is used to test the explosion limit of the gas mixture. Through this instrument, the gas can be automatically dispensed, and according to the flash fire phenomenon after ignition, it can be judged whether the sample gas has reached the explosion limit under the set concentration.
 Experimental video: (b) 30% concentration Experimental video: (b) 30% concentration |
|
 Experimental video: (d) 35% concentration Experimental video: (d) 35% concentration |
|
Due to gas volume limitations, a total of 5 experiments were conducted in this case and the results are summarized below:
|
Sample Gas Concentration |
20% |
30% |
40% |
35% |
32.5% |
|
Test Results |
Not ignited |
Not ignited |
Ignited and exploded |
Ignited and exploded |
Ignited and exploded |
Subsequent experimental validation was conducted using an Explosion Limit Test Instrument (HWP21-30S model). The instrument automatically mixes gases, and the gas concentration at which ignition occurs is indicative of reaching the explosion limit. The experiments were conducted multiple times due to gas volume limitations, and the results confirmed that the LFL range for the gas that evolved from the battery was between 32.5% and 35%.
Experimental Discussion – Large Battery Adiabatic Calorimeter
While the experimental results provide valuable insights, certain limitations exist. For instance, thermal runaway in lithium-ion batteries typically occurs in an inert gas atmosphere, but the introduction of a large amount of inert gas can increase the LFL of the gas evolved from the battery.
Additionally, the pressure conditions for explosion limit testing are not yet well-defined, and results may vary slightly between atmospheric pressure and high-pressure conditions (high-pressure testing requires the use of high-temperature and high-pressure explosion limit testing equipment). These issues warrant further discussion among industry experts to facilitate the establishment of relevant testing standards.