Accelerating Rate Calorimeter’s Exploration of Self-Decomposition in Airborne Samples
Introduction – Accelerating Rate Calorimeter’s
Typical thermal analyzers, such as adiabatic Accelerating Rate Calorimeter’s (ARC), Differential Scanning Calorimeter and Thermogravimetric Analyzer, have been widely used to study the thermal decomposition of chemicals. Typical thermal analyzers such as Differential Scanning Calorimeter (DSC) and Thermogravimetric Analyzer (TGA) have been widely used to study the process of thermally induced self-decomposition of chemicals, to obtain the heat of decomposition and decomposition kinetics data. However, these instruments face the same challenge in a special application scenario: for samples that are susceptible to hygroscopic reactions (e.g., metal-organic complexes, acrylates, etc.) or oxidative degradation (e.g., lithium battery cathode and negative electrode materials, phenolics, etc.) when exposed to the air, it is difficult to strictly avoid the samples from contacting with the air during sample preparation and loading unless the instrument and auxiliary equipments are placed in a glove box, and partial denaturation of samples will cause the test results to be inversely affected by the air. Partial denaturation will lead to a decrease in the accuracy and validity of the test results.
In this paper, we make full use of the structure of Accelerating Rate Calorimeter(TAC-500A) to study the thermal decomposition process of an aminotitanium substance, which is easy to be hydrolyzed by absorbing moisture, under nitrogen and air atmosphere respectively by designing a special way of loading samples. By comparing the thermal decomposition curves under the two conditions, it is proved that the initial decomposition temperature of the sample under the inert gas atmosphere is lower and the exothermic rate and heat of decomposition are higher, while the exothermic characteristics of the decomposition of the sample under the air atmosphere are obviously weakened. The results show that the method can effectively isolate the interference of air on the samples, which can provide a rigorous and effective way to study the self-decomposition characteristics of such special samples.
Experimental Conditions
- Sample: The study utilized amino titanium reagents as the sample under investigation.
- Instrument: Zeal Instruments’ Accelerating Rate Calorimeter(TAC-500A);
- Temperature Control Mode: Heat-Wait-Search (HWS);
- Sample Holder Type: Hastelloy alloy;
- Initial Temperature: 50°C;
- Target Temperature: 450°C;
- Ramp Rate: 5°C/min;
- Step Interval: 10°C;
- Temperature Rise Detection Threshold: 0.02°C.
Experimental Procedure
Nitrogen Atmosphere Experiment
- Weigh 0.6g of the sample and load it into the calorimetric bomb. Seal the bomb completely using a pressure-sealing tube at the sample introduction port within a nitrogen glovebox;
- Insert the thermocouple into the blind hole of the calorimetric bomb and pass the assembly through the ceramic fiber insulation layer from the bottom of the furnace lid;
- Connect the assembly to the four-way valve, close the instrument cover, set the experimental parameters, and commence the experiment.
Air Atmosphere Experiment
- Conduct sample loading conventionally outside the glovebox. Sample quantity remains 0.6g.
Experimental Results
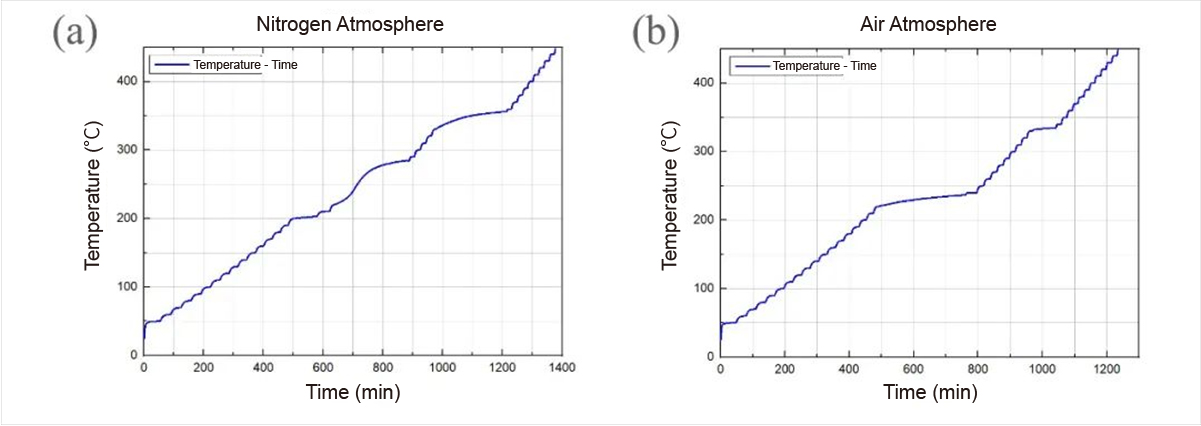
Fig. 1 Sample temperature versus time curves under (a) nitrogen and (b) air atmospheres
The temperature-time curves under nitrogen and air atmospheres, as depicted in Figure 1, exhibit distinct patterns. Under nitrogen atmosphere, three exothermic intervals are discernible compared to two under air atmosphere. Notably, exothermic peaks are more pronounced in nitrogen, suggesting possible sample moisture absorption and degradation in air.
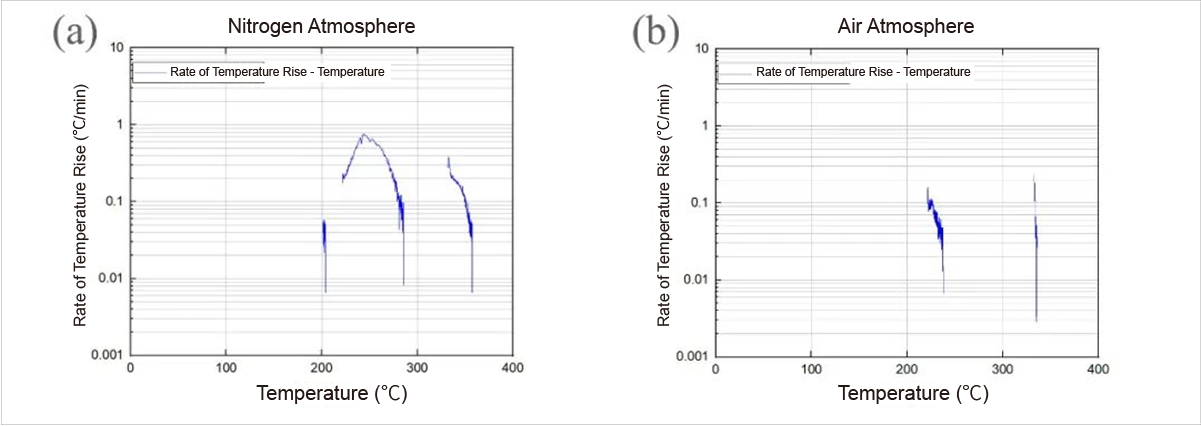
Fig. 2 Rate-temperature curves of sample temperature rise under (a) nitrogen and (b) air atmospheres
The temperature rise rate-temperature curves during adiabatic heating intervals, depicted in Figure 2, reveal significant differences between nitrogen and air atmospheres. The maximum temperature rise rate reaches 0.7°C/min under nitrogen compared to not exceeding 0.3°C/min under air, indicative of reduced reaction rates possibly due to sample degradation.
Comparative adiabatic data between nitrogen and air atmospheres (Table 1) elucidates consistent Tonset temperatures for the second and third exothermic stages but significantly reduced ΔTad under air, suggesting diminished participation of decomposing substances due to sample degradation.
Table 1 Comparison of adiabatic data for nitrogen and air atmosphere samples
|
Experiment |
First Exothermic |
Second Exothermic |
Third Exothermic |
|||
|
Tonset/℃ |
△Tonset/℃ |
Tonset/℃ |
△Tonset/℃ |
Tonset/℃ |
△Tonset/℃ |
|
|
Nitrogen Atmosphere |
200.13 |
2.04 |
220.29 |
64.34 |
330.59 |
25.35 |
|
Air Atmosphere |
– |
220.31 |
16.43 |
329.787 |
4.68 |
|
Conclusion
In conclusion, the Adiabatic Acceleration Calorimeter, exemplified by the TAC-500A from Zeal Instruments, represents a pinnacle in thermal analysis instrumentation. Through precise control of experimental parameters and adiabatic conditions, the TAC-500A facilitates in-depth exploration of chemical reactions’ thermal dynamics. Its versatility and accuracy make it an invaluable asset across various industries, empowering researchers to unravel complex phenomena and drive innovation forward.