Battery Adiabatic Calorimeter: A Comprehensive Guide
If you are involved in battery research and development, you know that safety is a top priority. Battery Adiabatic Calorimeters are a type of calorimeter designed to measure the thermal properties of batteries, particularly during thermal runaway. Thermal runaway is a condition where a battery becomes dangerously hot and could potentially explode. Adiabatic calorimeters are used to measure the heat generated during this process and can help researchers identify the causes of thermal runaway and develop safer batteries.
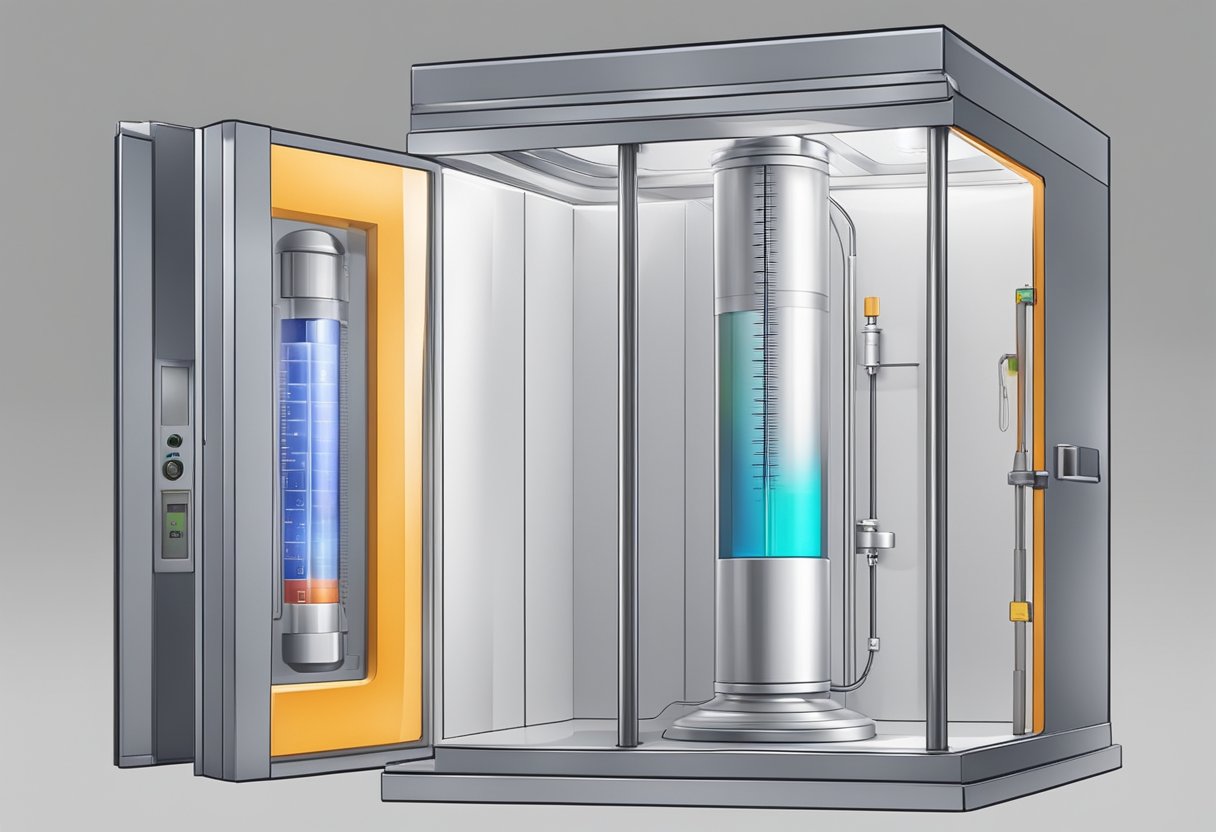
A battery adiabatic calorimeter is a bench-scale instrument that can measure the heat generated by a battery during thermal runaway. The instrument is designed to be adiabatic, meaning that it is thermally insulated, so that the heat generated by the battery is not lost to the environment. This allows researchers to accurately measure the heat generated by the battery and determine the thermal properties of the battery under different conditions.
Battery adiabatic calorimeters are used by researchers in a variety of fields, including battery development, safety testing, and performance evaluation. They are particularly useful for evaluating the safety of lithium-ion batteries, which are commonly used in portable electronic devices and electric vehicles. By measuring the thermal properties of these batteries, researchers can develop safer, more reliable batteries that are less likely to overheat and catch fire.
Fundamentals of Battery Adiabatic Calorimetry

If you are interested in understanding the thermal behavior of batteries, then you need to know about Battery Adiabatic Calorimetry. Adiabatic calorimetry is a technique that measures the heat generated or absorbed during a chemical reaction or physical change under adiabatic conditions. Battery Adiabatic Calorimetry is a type of adiabatic calorimetry that is used to evaluate the thermal behavior of batteries.
The Battery Adiabatic Calorimeter is a device that measures the heat generated or absorbed by a battery under adiabatic conditions. It is used to evaluate the thermal behavior of batteries, including the heat generated during charging and discharging, as well as the heat generated during thermal runaway. Thermal runaway is a phenomenon that occurs when a battery overheats and generates heat faster than it can dissipate. This can lead to a catastrophic failure of the battery, including an explosion or fire.
Battery Adiabatic Calorimetry is an essential tool in battery research and development, as well as in battery safety testing. It is used to evaluate the thermal behavior of batteries under various operating conditions, including different charging and discharging rates, different temperatures, and different states of charge.
The Battery Adiabatic Calorimeter is typically composed of a cell holder, a temperature sensor, and a pressure sensor. The cell holder is used to hold the battery under test, while the temperature sensor is used to measure the temperature of the battery. The pressure sensor is used to measure the pressure inside the cell holder, which is an indication of the heat generated by the battery.
In conclusion, Battery Adiabatic Calorimetry is a powerful tool for understanding the thermal behavior of batteries. It is used to evaluate the heat generated or absorbed by batteries under adiabatic conditions, including the heat generated during charging and discharging, as well as the heat generated during thermal runaway. The Battery Adiabatic Calorimeter is an essential tool in battery research and development, as well as in battery safety testing.
Thermal Hazard Evaluation
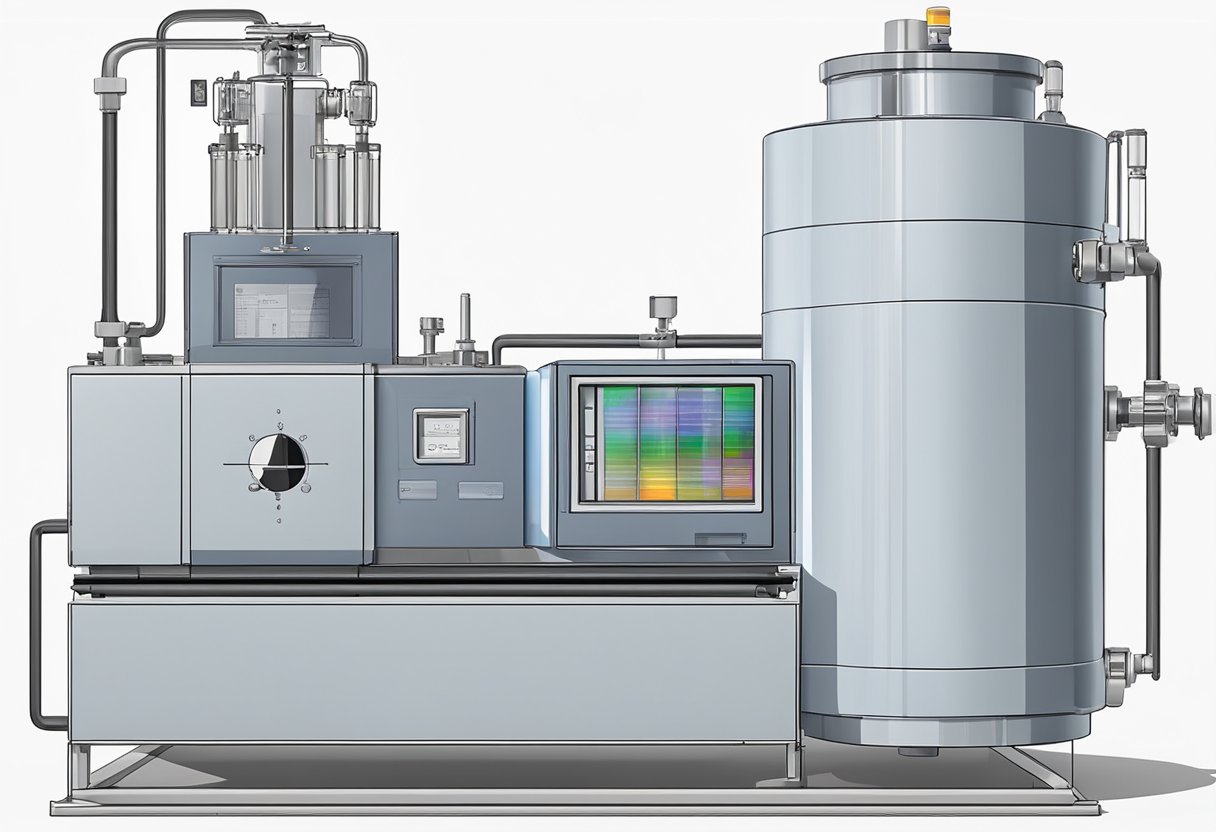
When it comes to evaluating the thermal hazards of batteries, the adiabatic calorimeter is a crucial tool. This device is capable of measuring the heat flow and temperature changes of a battery when it is subjected to an adiabatic condition. In this section, we will explore the two main aspects of thermal hazard evaluation using an adiabatic calorimeter: thermal runaway detection and heat flow measurement.
Thermal Runaway Detection
Thermal runaway is a phenomenon that occurs when a battery experiences an exothermic reaction that generates heat faster than it can dissipate it. This can lead to a chain reaction that causes the battery to heat up rapidly, potentially causing an explosion or fire. Adiabatic calorimeters are capable of detecting thermal runaway by measuring the temperature and pressure changes that occur when a battery is subjected to an adiabatic condition. This information can be used to identify the conditions that lead to thermal runaway and to develop strategies to prevent it from occurring.
Heat Flow Measurement
Heat flow measurement is another important aspect of thermal hazard evaluation using an adiabatic calorimeter. This involves measuring the heat flow that is generated by a battery when it is subjected to an adiabatic condition. This information can be used to determine the thermal properties of the battery, such as its specific heat capacity and thermal conductivity. This information can be used to design batteries that are more resistant to thermal hazards and to develop strategies to prevent thermal runaway.
In summary, thermal hazard evaluation using an adiabatic calorimeter is an essential tool for understanding the thermal properties of batteries. By measuring the temperature and pressure changes that occur during thermal runaway and the heat flow generated by the battery, it is possible to develop strategies to prevent thermal hazards and to design safer batteries.
Instrumentation and Setup
Calorimeter Components
The adiabatic battery calorimeter consists of several components that work together to measure the heat generated by a battery during a thermal runaway event. These components include:
-
Calorimeter Vessel: This is the main component of the calorimeter and is made of a thermally conductive material such as aluminum. The vessel is designed to be adiabatic, which means that heat cannot escape from the vessel during a thermal runaway event.
-
Pressure Transducer: The pressure transducer measures the pressure changes inside the calorimeter vessel during a thermal runaway event. The pressure changes are directly related to the heat generated by the battery.
-
Temperature Sensors: The temperature sensors are used to measure the temperature inside the calorimeter vessel during a thermal runaway event. These sensors are placed at different locations inside the vessel to ensure accurate temperature measurements.
-
Gas Sampling System: The gas sampling system is used to collect gas samples from the calorimeter vessel during a thermal runaway event. These gas samples are analyzed to determine the composition of the gases generated by the battery.
Sample Preparation
Before conducting a thermal runaway test, the battery must be prepared according to specific guidelines. The following steps are typically followed:
-
Battery Selection: The battery to be tested is selected based on its size, chemistry, and other relevant characteristics.
-
Battery Conditioning: The battery is conditioned to a specific state of charge and temperature before the test. This ensures that the battery is in a stable state before the test begins.
-
Battery Installation: The battery is installed in the calorimeter vessel according to specific guidelines. The installation process must be carefully followed to ensure accurate test results.
-
Test Initiation: The thermal runaway test is initiated by applying a specific heat flux to the battery. The heat flux is carefully controlled to ensure that the battery does not experience a thermal shock.
In summary, the adiabatic battery calorimeter is an essential tool for measuring the heat generated by a battery during a thermal runaway event. The calorimeter consists of several components that work together to ensure accurate test results. Additionally, the battery must be carefully prepared before the test to ensure accurate and reliable results.






































