Accelerating Rate Calorimeter for Analysing Thermal Stability of Battery Materials

Overview of Accelerating Rate Calorimeter for Analysing Thermal Stability
In the relentless pursuit of improved battery technologies, researchers face a dual challenge: enhancing energy density and ensuring safety. The significance of battery cell development cannot be overstated, particularly in an era where electric vehicles, portable electronics, and renewable energy storage systems rely heavily on lithium-ion batteries. However, alongside advancements in performance, safety optimization remains paramount. The Accelerating Rate Calorimeter (ARC), manufactured by Zeal Instruments, emerges as a vital tool in this endeavor, offering precise insights into the thermal stability of battery materials.
Introduction
The landscape of battery cell development is fraught with challenges, prominently including achieving higher energy densities, extending cycle life, and addressing safety concerns. While advancements have been made, the quest for higher energy density often involves pushing the boundaries of materials, which can inadvertently exacerbating safety risks. Thermal runaway, a phenomenon characterized by uncontrolled temperature increase, remains a significant concern. Hence, safety optimization strategies are indispensable to mitigate such risks and ensure the widespread adoption of lithium-ion batteries across various applications.
At present, there are two main methods for testing the thermal stability of battery materials as follows [1]:
- DSC test method: the sample heating power is calculated by measuring the power difference per unit time between the sample and the reference under program temperature control or the temperature difference with temperature change. This method uses milligram-level sample volumes and has high accuracy in testing homogeneous systems, but the test results may lack statistical significance for non-homogeneous systems such as electrode materials and electrolyte mixtures;
- ARC test method: The change in the rate of temperature rise of the sample is measured in an adiabatic environment, so that the heating power of the sample can be calculated from the adiabatic temperature rise per unit time. This method is a gram-scale test and is therefore more suitable for the determination of non-homogeneous samples.
In this paper, the characteristic parameters of the thermal decomposition reaction of battery materials were determined using the classical HWS mode of an adiabatic accelerating rate calorimeter (Accelerating Rate Calorimeter), and the kinetic parameters of the reaction were obtained by fitting based on the Arrhenius equation . The relevant results are helpful for battery design and system thermal simulation to improve the thermal safety performance of battery systems.
Experimental Insights
Sample Preparation
Samples included nickel cathode materials at 50% state of charge (SOC) and the electrolyte (EC+DMC+LiPF6). The experiments were conducted in the adiabatic environment of the ARC, ensuring precise measurements.
Experimental Conditions
Experimental Instruments: Accelerating Rate Calorimeter (TAC-500A);
Operating mode: HWS mode;
Calorimetric bomb: volume 8mL, Hastelloy (specific heat 0.425Jg-1-k-1);
Glove box atmosphere replacement vacuum: -0.085MPa;
Glove box protective atmosphere: nitrogen;
Glove box atmosphere replacement times: 3 times.
Table 1 Parameter table for setting experimental conditions
|
Sample number and name |
Calorimetric bomb mass (g) |
Sample mass (g) |
Test mode |
Temperature between start-up intervals |
Constant time between start-up intervals |
Step |
Temperature of bench step |
Temperature at the end of the experiment |
|
#0 Quartz Shafts |
– |
– |
Temperature Baseline |
50 |
45 |
25 |
45 |
500 |
|
#1 50%SOC medium nickel cathode material+electrolyte |
25.62 |
3.06 (1.81g+0.996ml) |
HWS |
50 |
45 |
5 |
45 |
500 |
|
#2 50%SOC high nickel cathode material+electrolyte |
25.87 |
2.42 (1.44g+0.792ml) |
HWS |
50 |
45 |
5 |
45 |
500 |
|
#3 50%SOC high nickel cathode material |
25.93 |
2.06 |
HWS |
50 |
45 |
5 |
45 |
500 |
|
#4 Electrolyte |
25.95 |
2.33 |
HWS |
50 |
45 |
5 |
45 |
500 |
Test Procedure
Weigh the calorimetric bomb in the glove box, and add a certain amount of sample, followed by a cut-off pressure tube and joint group welding sealed calorimetric bomb; as shown in Figure 2, the calorimetric bomb assembly is installed to to the adiabatic accelerated calorimetry; set up the experimental parameters (see Table 1), and then turn on the experiment.
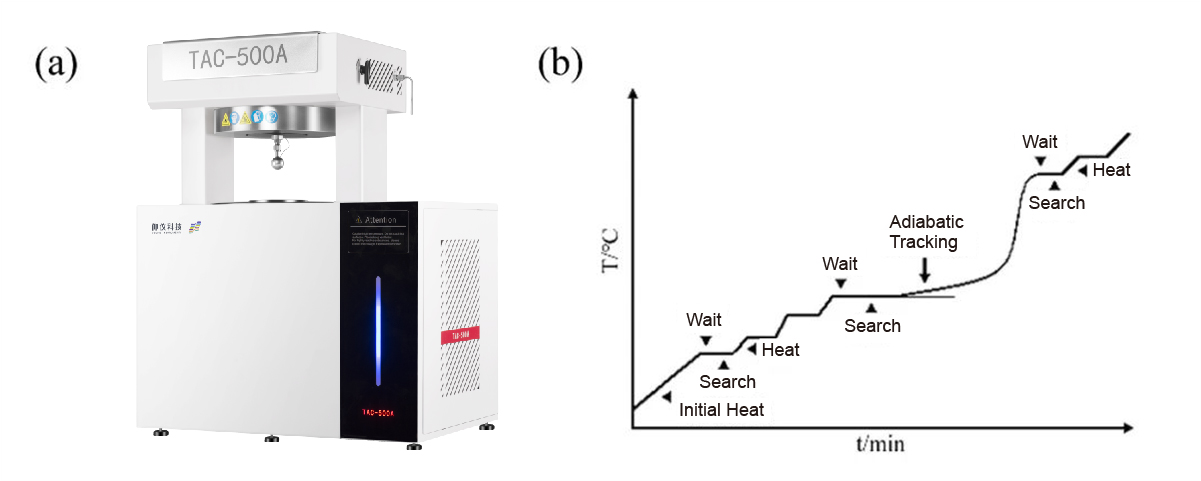 Fig. 2 Accelerating Rate Calorimeter(TAC-500A)b) Schematic diagram of the HWS model principle
Fig. 2 Accelerating Rate Calorimeter(TAC-500A)b) Schematic diagram of the HWS model principle
Experimental Results
High Nickel Cathode Materials, Electrolyte and Mixtures of the Two
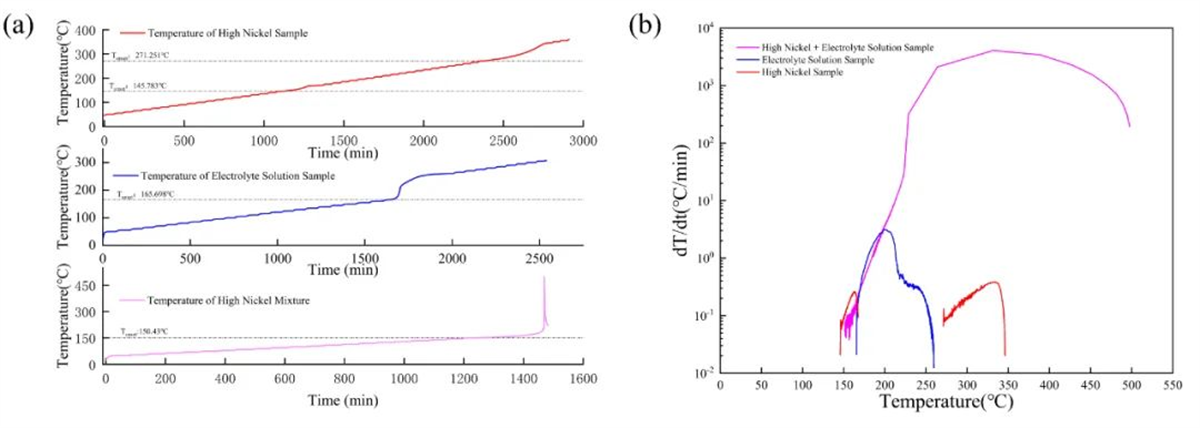
Fig. 3 HWS (a) temperature rise curves and (b) temperature rise rate-temperature curves for high nickel cathode materials, electrolyte and mixtures of the two
The experiments revealed distinct thermal decomposition behaviors of the cathode materials, electrolyte, and their mixtures. For instance, the onset temperature for self-heating differed between cathode materials and electrolytes, with the mixture falling in between. Additionally, violent reactions were observed between high -nickel cathode materials and the electrolyte, highlighting potential safety concerns.
Different Cathode Materials and Electrolyte Mixtures
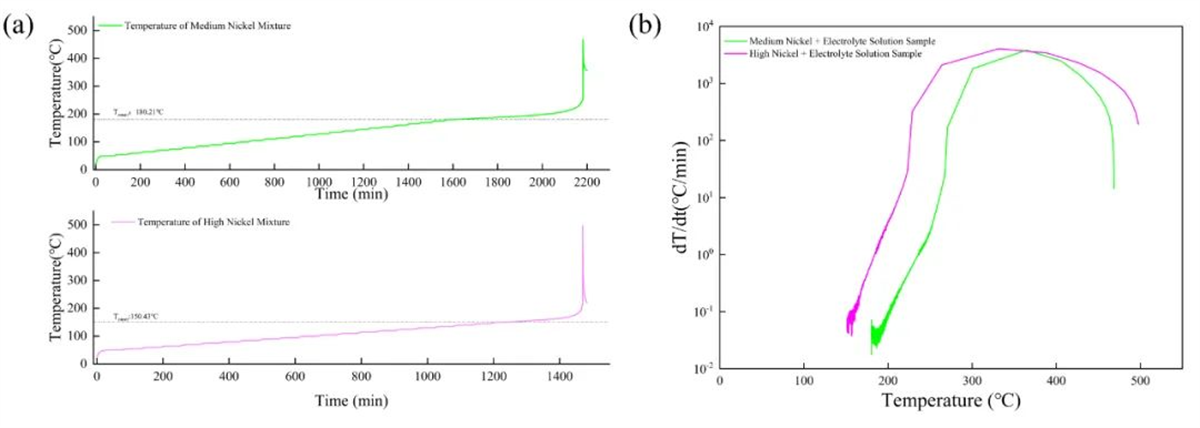
Fig. 4 HWS (a) temperature rise curves and (b) temperature rise rate-temperature curves for different cathode materials and electrolyte mixtures
As shown in Fig. 4 and Table 2, the self-exothermic onset temperature of the nickel cathode material & electrolyte mixture in 50% SOC is higher than the values of the high-nickel material & electrolyte, and its maximum temperature of reaction and maximum rate of temperature rise are lower than the values of the high-nickel material & electrolyte, which proves that the thermal stability of the high-nickel cathode is relatively low.
Table 2 Summary of thermodynamic parameters
|
Sample number and name |
Reaction onset temperature |
Maximum reaction temperature |
Maximum rate of temperature rise |
Reaction heat release per unit mass |
Thermal inertness factor |
|
#1 50%SOC medium nickel cathode material+electrolyte |
180.21 |
468.59 |
378.37 |
1178.35 |
2.04 |
|
#2 50%SOC high nickel cathode material+electrolyte |
150.43 |
497.72 |
485.37 |
1625.91 |
2.34 |
|
#3 50%SOC high nickel cathode material |
145.78 |
167.58 |
0.26 |
112.54 |
2.58 |
|
271.25 |
345.92 |
0.38 |
385.53 |
||
|
#4 Electrolyte |
165.70 |
260.18 |
3.15 |
453.51 |
2.40 |
Kinetic Parameter Fitting
Leveraging the data obtained from the ARC, researchers performed kinetic parameter fitting using software analysis modules. This yielded optimized results, providing valuable equations for reaction kinetics and enhancing battery system thermal simulations.
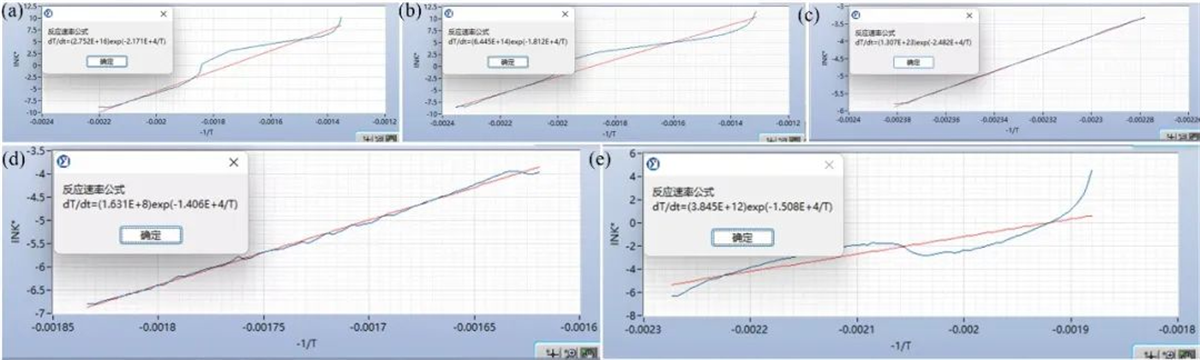
Fig. 5 Kinetic fitting results of thermal decomposition reactions for (a) 50%SOC medium nickel cathode material & electrolyte mixture, (b) 50%SOC high nickel cathode material & electrolyte mixture, (c,d) 50%SOC high nickel cathode material & electrolyte mixture material, and (e) electrolyte
Table 3 Summary of kinetic parameters
|
Sample number and name |
Optimum grade |
Activation energy E(J/mol) |
Finger forward factor Aa(s-1) |
|
#1 50%SOC medium nickel cathode material+electrolyte |
2.30 |
1.805E+5 |
4.587E+14 |
|
#2 50%SOC high nickel cathode material+electrolyte |
3.00 |
1.507E+5 |
1.074E+13 |
|
#3 50%SOC high nickel cathode material |
0.50 |
2.06E+5 |
2.18E+21 |
|
0.60 |
1.17E+5 |
2.76E+6 |
|
|
#4 Electrolyte |
3.00 |
1.25E+5 |
6.41E+10 |
Conclusion
In conclusion, the Accelerating Rate Calorimeter (ARC) represents a cornerstone technology in the realm of battery research and safety optimization. Its ability to provide precise measurements of thermal stability parameters empowers researchers to navigate the complexities of material development with confidence. As the demand for high-performance, safe lithium-ion batteries continue to soar, the ARC’s role in shaping the future of energy storage remains indispensable.
Reference
[1] Feng X, Zheng S, Ren D, et al. Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal analysis database[J]. Applied energy, 2019, 246: 53-64.
[2] Wang Q, Sun J, Yao X, et al. Thermal stability of LiPF6/EC+ DEC electrolyte with charged electrodes for lithium ion batteries[J]. Thermochimica Acta, 2005, 437(1-2): 12-16.
[3] Röder P, Baba N, Friedrich KA, et al. Impact of delithiated Li0FePO4 on the decomposition of LiPF6-based electrolyte studied by accelerating rate calorimetry[J]. Journal of power sources, 2013, 236: 151-157.