Principle and Application Fields of Auto Bomb Calorimeter
The auto oxygen bomb calorimeter measures the heat of combustion by placing the substance in a sealed container under high-pressure oxygen.
The fundamental principle of the oxygen bomb calorimeter involves placing a substance to be analyzed and sufficient oxygen inside the calorimeter, surrounded by a water container. The substance is ignited using cotton thread or similar means, and the heat released from the combustion raises the temperature of the water. The increase in water temperature is measured to determine the combustion heat of the reaction.
High and Low Heating Values
The combustion heat measured by the Auto Bomb Calorimeter can be classified into two types: high heating value and low heating value. The high heating value refers to the heat content calculated assuming the combustion products are cooled to the initial temperature and the water in the products is in liquid form (condensed).
Low Heating Value
In contrast, the low heating value calculates the heat content with the water in the combustion products remaining in steam form at the initial temperature. The low heating value is the high heating value minus the heat of condensation of the water vapor.
International Usage Standards
While a few countries like Japan and the United States use the high heating value for energy calculations, most countries base their energy utilization calculations on the low heating value. This standardization helps in providing a consistent basis for energy measurement and comparison across different regions and substances.
Introduction to the Oxygen Bomb Calorimeter + Technical Specifications
Advanced Features
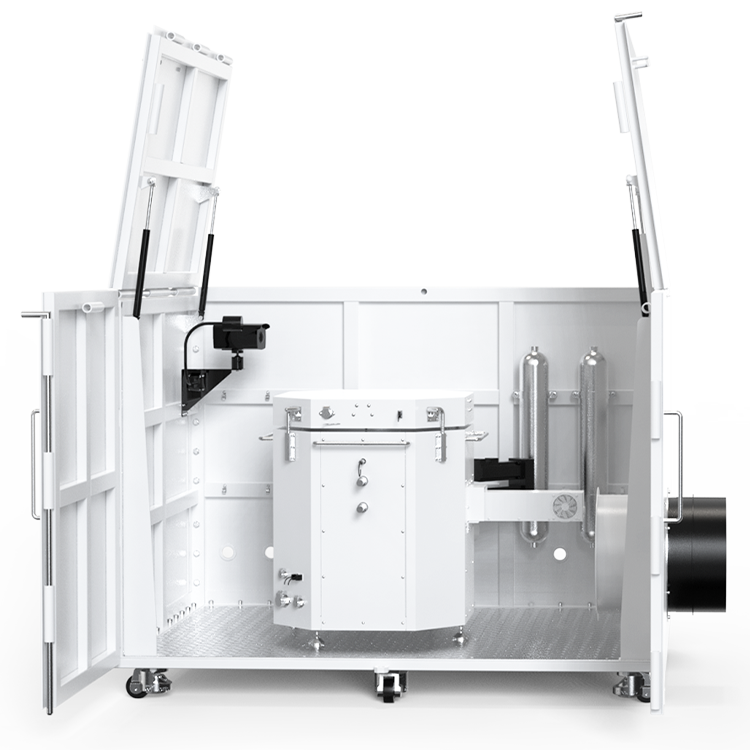
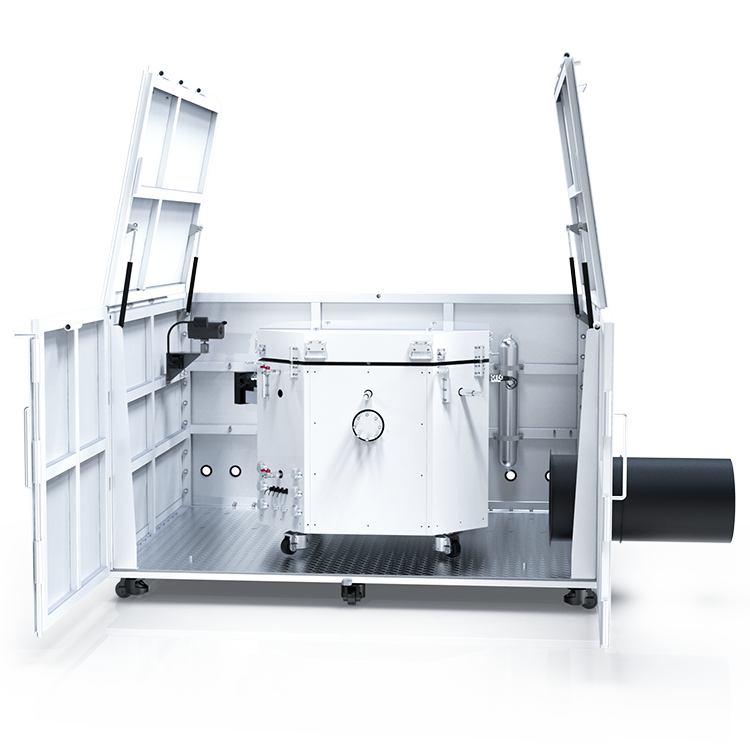
The Oxygen Bomb Calorimeter(ATC 300A ), adhering to standards such as GB/T384, GB/T 213, ASTM 4809, and ASTM D240, is designed for rapid and precise calorific value determination of combustibles. This device features an automatic handling system for bomb lifting, serial number recognition, oxygen filling and release, and oxygen pressure detection, which significantly enhances the ease of operation. The system’s high-precision temperature control ensures both accuracy and repeatability in test results, while its user-friendly interface simplifies the testing process, making it accessible even for novice users.
Enhanced Operational Efficiency and Safety
The ATC 300A is equipped with a semiconductor cooling water circulation system, complete with a high-efficiency filter that monitors and controls water temperature across heating, insulation, and cooling phases, swiftly achieving thermal equilibrium. This minimizes environmental interference during tests. The calorimeter also features automatic ignition wire detection and short-circuit protection, enhancing both safety and reliability. The touchscreen color LCD not only facilitates stable software operation but also displays real-time data curves, making the testing process more intuitive and informative.
Automated Data Management and Calibration
This calorimeter automatically corrects data to provide three types of calorific values: higher heating value, lower heating value, and bomb calorific value, adjusting for variables such as ignition wire combustion and the calorific value of additional chemical components. It supports advanced serial port technology for multi-control operations, allowing multiple units to run simultaneously without interference. Additionally, it automatically generates and saves charts and process data, which can be retrieved for historical analysis, enhancing the research and quality control processes.
Dual Control System and Robust Design
The ATC 300A integrates a dual control system that combines a color touchscreen with a PC-based operating system, enabling synchronized monitoring of experiments. This integration not only enhances the safety and convenience of the device but also ensures a more reliable and user-friendly experience. The calorimeter operates optimally within a temperature range of 15°C to 30°C and can handle up to 80% relative humidity without condensation. Its robust design features materials like stainless steel and Hastelloy, offering resistance to corrosion and ensuring long-term durability and performance.

Diverse Applications of Oxygen Bomb Calorimeters
Application in Coal Calorific Value Determination
The calorific value of raw coal is a critical quality indicator, widely measured in the power and chemical industries using oxygen bomb calorimeters. Factors like oxygen filling pressure, duration, and the temperature of both inner and outer cylinders significantly influence the accuracy of these measurements.
Biomass Calorific Value Analysis
The oxygen bomb calorimeter is also utilized in determining the calorific value of common legumes, aiding in diet optimization and nutritional improvement.
Pre-treatment Technique for Sample Analysis
As a pre-treatment technology, the oxygen bomb is used for combustion-ion chromatography to determine chlorine content in infant formula. By burning the milk powder in an oxygen-rich sealed environment, all chlorine elements are converted to inorganic chloride ions, improving the accuracy and reproducibility of ion chromatography analyses.
Synthesis of Inorganic Nanomaterials
The high-temperature combustion environment of the oxygen bomb is exploited in the synthesis of potassium titanate, a high-performance inorganic fiber material. Traditional synthesis methods take hours to days, but using the oxygen bomb, the synthesis can be completed in just minutes under oxygen-rich conditions. This method not only reduces the reaction time but also allows for the study of reaction mechanisms based on the combustion heat values obtained under different conditions.
Precautions for Measuring Coal Calorific Value with an Oxygen Bomb Calorimeter
Importance of Proper Sample Mixing Before Measurement
When measuring the calorific value of coal, achieving a uniform mix of the coal sample in the container is crucial due to its heterogeneous nature in grain size and chemical composition. Inadequate mixing can lead to significant errors in the results due to the natural segregation of grains by size during handling and storage, with larger grains settling at the bottom and sides and finer grains at the top and center. To ensure accurate measurements, the “bottle rotation method” should be used for thorough mixing. This involves sealing the sample container, holding the cap with one finger, and rotating the wrist to mix the sample in all directions until there are no visible coal dust clouds before weighing. Unfortunately, many laboratory technicians simply stir the sample with a spoon and proceed to weigh it, reducing the representativeness and precision of the results.
Pre-use Inspection of Combustion Vessels
Before any experiment, it is essential to check the combustion vessel for any deformations or thinning at the bottom. Extended use or exposure to high-sulfur, high-calorific coal samples can cause the bottom to deform and thin out, potentially melting upon ignition and causing a short circuit that can damage the oxygen bomb. Additionally, the oxygen bomb must be checked for airtightness by submerging the oxygen-filled vessel in water; the presence of continuous bubbles indicates a leak and necessitates repair before use. Leakage usually results from poor connections or worn parts, such as elastic seals that have lost their flexibility due to aging or dryness.
CONCLUSION Auto Bomb Calorimeter
The auto oxygen bomb calorimeter operates on the principle of measuring the heat released during a sample’s combustion in a high-pressure oxygen environment. This precise calorimetric analysis is crucial in various fields. In the energy sector, it evaluates the calorific value of fuels like coal, oil, and biofuels, ensuring efficiency and quality. The food industry uses it to determine the energy content of food products, contributing to nutritional analysis. Environmental studies benefit from its use in analyzing waste and biomass, aiding in sustainable practices. Additionally, the chemical industry relies on it for quality control and research. The auto oxygen bomb calorimeter’s versatility and accuracy make it an indispensable tool across these diverse application fields.