Battery Performance Calorimeter: Understanding Its Importance in Battery Testing
If you’re interested in studying the thermal behavior of batteries, a Battery Performance Calorimeter (BPC) is an essential tool to have. The BPC is a large volume calorimeter that is specifically designed for research studies of larger electric vehicle (EV) cells and modules. It is used to quantify heat changes during charge and discharge at conditions that simulate the use of the battery.

The BPC is capable of measuring the heat generated by a battery during operation, which is a critical factor in determining battery performance and safety. This information is useful for researchers and manufacturers who need to understand how a battery will perform in different environments and under different conditions. The BPC can also be used to assess the safety of batteries by measuring the heat generated during abuse tests, such as puncture or overcharge.
Overall, the BPC is an essential tool for anyone involved in battery research and development. It provides valuable information about battery performance and safety, which can be used to improve battery design and manufacturing processes.
Fundamentals of Battery Performance Calorimetry

Principles of Calorimetry
Battery Performance Calorimetry is a technique used to measure the thermal behavior of batteries during charge and discharge cycles. The principle of calorimetry is based on the measurement of heat flow in and out of a system. In the case of battery calorimetry, the heat generated or absorbed during charge and discharge cycles is measured to evaluate the thermal behavior of the battery.
Calorimetry can be performed using various types of calorimeters, such as isoperibol, bomb, and differential scanning calorimeters. The choice of calorimeter depends on the type of battery and the specific application. For example, isoperibol calorimeters are commonly used for measuring the heat capacity of batteries, while bomb calorimeters are used for measuring the heat of combustion of batteries.
Battery Performance Metrics
Battery Performance Calorimetry provides valuable information about the thermal behavior of batteries, which is essential for evaluating battery performance and safety. The following are some of the key metrics that can be obtained using battery calorimetry:
-
Heat Capacity: The heat capacity of a battery is the amount of heat required to raise the temperature of the battery by one degree Celsius. Heat capacity is an important parameter for designing battery thermal management systems.
-
Heat Generation: The heat generated during charge and discharge cycles is an important parameter for evaluating battery performance and safety. High heat generation can lead to thermal runaway and safety hazards.
-
Heat Dissipation: The rate at which heat is dissipated from a battery is an important parameter for evaluating the effectiveness of battery thermal management systems.
-
Thermal Runaway: Thermal runaway is a condition where the heat generated by a battery during charge and discharge cycles exceeds the heat dissipation rate, leading to an uncontrolled increase in temperature and potential safety hazards. Battery calorimetry can be used to evaluate the thermal runaway behavior of batteries and to design safety mechanisms to prevent thermal runaway.
In conclusion, Battery Performance Calorimetry is a valuable technique for evaluating battery performance and safety. By measuring the thermal behavior of batteries during charge and discharge cycles, important metrics such as heat capacity, heat generation, heat dissipation, and thermal runaway can be obtained, which are essential for designing battery thermal management systems and ensuring battery safety.
Calorimeter Design for Battery Testing
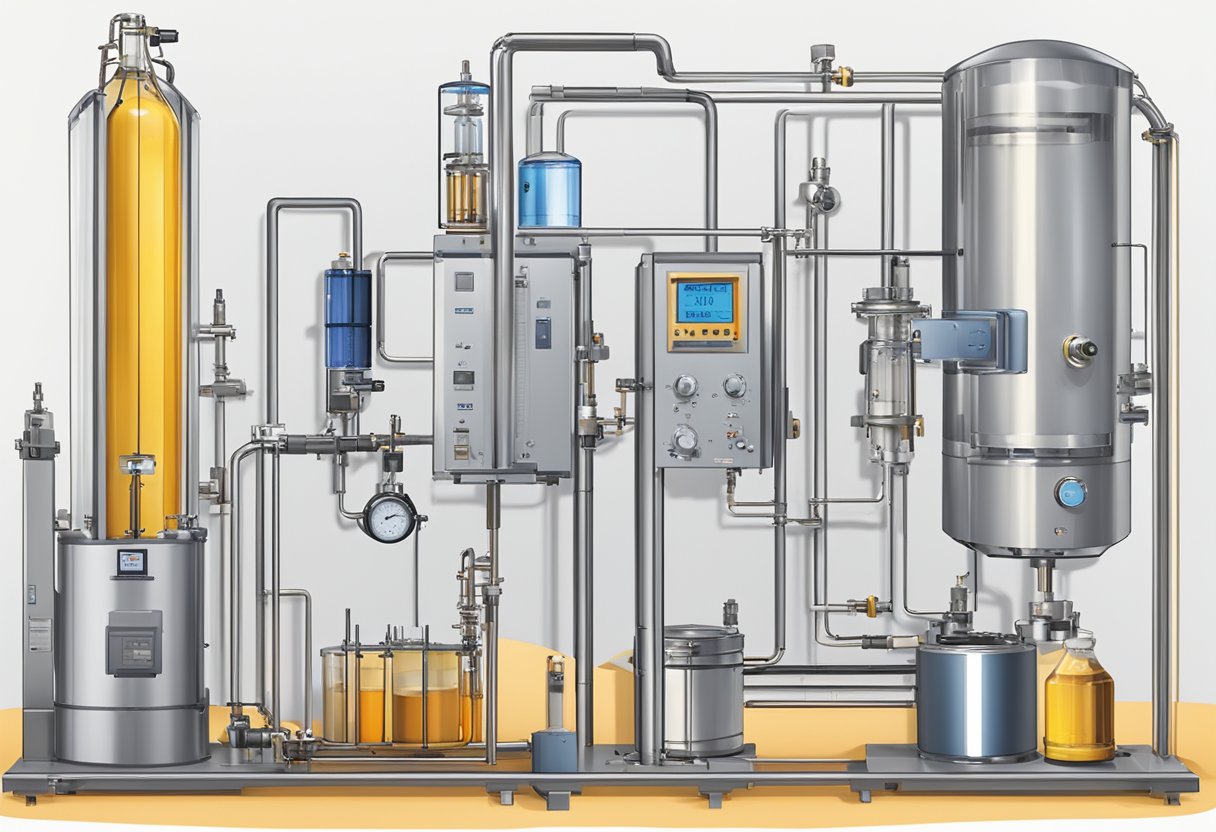
When it comes to testing batteries, a calorimeter is an essential tool. Calorimeters are designed to measure the heat generated by a battery during operation, which is a critical factor in determining the battery’s performance and safety. In this section, we will discuss the design of a calorimeter for battery testing.
Thermal Management
One of the most critical aspects of a battery calorimeter is thermal management. Batteries generate a significant amount of heat during operation, and if this heat is not managed correctly, it can lead to safety issues. Therefore, a calorimeter must be designed to manage the heat generated by the battery effectively.
There are two primary methods for managing the heat generated by a battery: adiabatic and isothermal. Adiabatic calorimeters are designed to measure the heat generated by a battery under conditions where no heat is allowed to escape from the system. In contrast, isothermal calorimeters are designed to measure the heat generated by a battery under constant temperature conditions.
Measurement Accuracy
Another critical factor in calorimeter design is measurement accuracy. The accuracy of a calorimeter is essential because it determines the reliability of the data generated by the calorimeter. Calorimeters must be designed to measure the heat generated by a battery accurately.
One way to improve measurement accuracy is to use a calorimeter with a high-resolution temperature sensor. A high-resolution temperature sensor can detect small changes in temperature, which can improve the accuracy of the data generated by the calorimeter.
Another way to improve measurement accuracy is to use a calorimeter with a high-precision heat flow sensor. A high-precision heat flow sensor can measure the heat generated by a battery with high accuracy, which can improve the reliability of the data generated by the calorimeter.
In conclusion, the design of a calorimeter for battery testing is critical for ensuring the reliability and safety of batteries. A calorimeter must be designed to manage the heat generated by the battery effectively and measure the heat generated by the battery accurately.
Battery Performance Testing Protocols
Standard Test Procedures
When it comes to battery performance testing, there are several standard test procedures that are commonly used. These procedures are designed to ensure that batteries are tested in a consistent and repeatable manner, so that results can be compared across different tests and different batteries. Some of the most common test procedures include:
-
Constant Current Discharge Test: This test involves discharging the battery at a constant current until it reaches a specified endpoint voltage. This test is often used to determine the capacity of a battery.
-
Pulse Discharge Test: This test involves discharging the battery in short pulses, with a rest period between each pulse. This test is often used to simulate the high-current demands that batteries may experience in real-world applications.
-
Cycle Life Test: This test involves cycling the battery through a series of charge and discharge cycles, while monitoring its capacity and other performance parameters. This test is often used to determine the expected lifespan of a battery.
Data Analysis Techniques
Once the battery performance testing is complete, the data must be analyzed in order to draw meaningful conclusions about the battery’s performance. There are several data analysis techniques that are commonly used, including:
-
Capacity Analysis: This involves analyzing the amount of charge that the battery is able to store and deliver over time. This analysis can be used to determine the battery’s overall capacity, as well as its ability to maintain capacity over multiple charge and discharge cycles.
-
Voltage Analysis: This involves analyzing the voltage of the battery over time, and can be used to determine the battery’s state of charge and state of health.
-
Impedance Analysis: This involves analyzing the electrical impedance of the battery, and can be used to determine the battery’s internal resistance and other electrical properties.
By using these standard test procedures and data analysis techniques, battery performance can be accurately and consistently evaluated, allowing for informed decisions to be made about battery selection and usage.
Applications of Battery Calorimeters
Battery calorimeters are widely used in the battery industry for various applications such as research and development, quality control, and safety testing. In this section, we will discuss the two main applications of battery calorimeters.
Research and Development
Battery calorimeters are an essential tool for battery research and development. They are used to study the thermal behavior of batteries, which is crucial for improving battery performance and safety. Battery calorimeters can measure the heat generated by batteries during charging and discharging, which helps researchers understand the battery’s energy efficiency and capacity.
Battery calorimeters are also used to study the effects of temperature on battery performance. By varying the temperature of the battery, researchers can determine the optimal temperature range for the battery to operate in. This information is critical for developing batteries that can perform reliably in extreme temperatures.
Quality Control
Battery calorimeters are also used in quality control to ensure that batteries meet specific performance standards. By measuring the heat generated by batteries during charging and discharging, manufacturers can verify that the battery’s energy efficiency and capacity meet the required specifications.
Battery calorimeters are also used to detect defects in batteries. For example, if a battery is overcharged, it can generate excessive heat, which can cause the battery to fail. By measuring the heat generated by the battery during charging, manufacturers can identify defective batteries before they are shipped to customers.
In summary, battery calorimeters are a critical tool for battery research and development and quality control. By measuring the heat generated by batteries during charging and discharging, battery calorimeters provide valuable information about battery performance and safety.
Advancements in Calorimetry Technology
Calorimetry technology has come a long way in recent years, with advancements in sensing elements, software, and data integration. These advancements have led to more accurate and efficient testing of batteries, allowing for better performance and safety.
Innovations in Sensing Elements
One of the most significant advancements in calorimetry technology has been the development of new sensing elements. These elements are designed to provide more accurate and detailed information about the thermal behavior of batteries.
For example, some sensing elements are now capable of measuring the temperature of individual battery cells, allowing for more precise monitoring of battery performance. Other sensing elements can detect changes in pressure, which can be an indication of a potential safety issue.
Software and Data Integration
Another area of innovation in calorimetry technology is software and data integration. With the development of new software tools, it is now possible to collect and analyze data from multiple sources in real-time.
This allows for more efficient testing and analysis of battery performance, as well as the identification of potential safety issues before they become a problem. Additionally, the integration of data from multiple sources can provide a more comprehensive picture of battery behavior, leading to better overall performance.
In conclusion, advancements in calorimetry technology have led to more accurate and efficient testing of batteries, allowing for better performance and safety. Innovations in sensing elements and software and data integration have played a significant role in these advancements, providing researchers with more detailed information about battery behavior.