Comparative Study of Thermal Runaway Catalytic Reaction Models for Liquid and Semi-Solid Electrolyte Batteries
In the context of the global active promotion of carbon peak and carbon neutrality, the new energy vehicle industry, as a key element to achieve carbon peak and carbon neutrality, has become increasingly strategically significant. As the power source of new energy vehicles, the energy density of lithium-ion batteries is closely related to the vehicle’s range, weight, and cost, but as the energy density increases, the risk and severity of thermal runaway also increase.
In order to deeply analyze the thermal runaway characteristics of high-energy-density batteries and provide a scientific basis for the design of electric vehicle thermal management systems, our company’s application research team cooperated with the research group of Associate Professor Lin Chunjing of Chongqing University of Technology to use the BAC-420A large battery adiabatic calorimeter to conduct adiabatic thermal runaway tests on ternary lithium-ion liquid batteries and lithium metal semi-solid batteries, and compared the catalytic reactions during the thermal runaway process. The relevant research results have been published in the Journal of Energy Storage.
Experimental Instrument
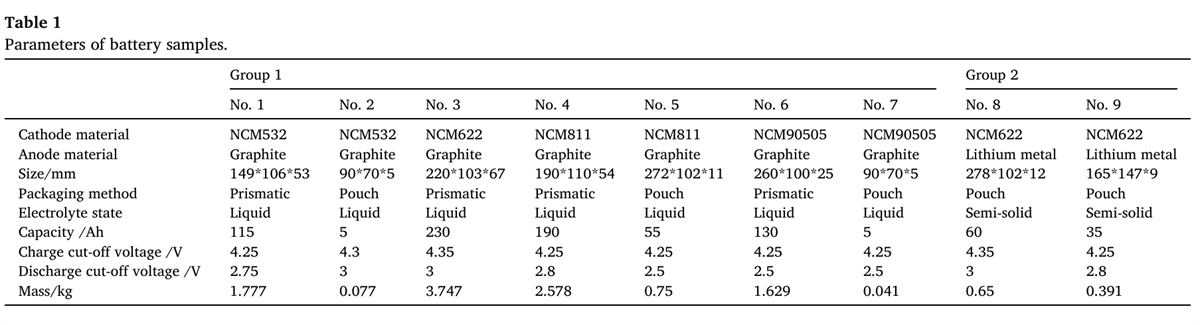
The large battery adiabatic calorimeter, as a key device for battery thermal safety assessment, can precisely detect the thermal runaway characteristics of batteries in an adiabatic environment. It synchronously records the voltage, current, temperature, and time status information of batteries under various abuse conditions. Through the collaborative processing of electrical, thermal, and optical data, it reveals the mechanism of battery thermal runaway, accurately quantifies battery thermal stability and disaster-causing hazards, and provides reliable data support for the safety performance assessment of battery cells and modules, thermal management development, and active prevention and control of thermal runaway.
The BAC-420A large battery adiabatic calorimeter used in this study is specially designed for large battery cells and small modules. The effective size of its adiabatic chamber is 420×520mm, which meets the requirements for battery thermal runaway detection with a long side not exceeding 600mm. The instrument has good thermal insulation performance, the wall sample temperature difference is ≤0.5℃, and the self-heating detection sensitivity is 0.005℃/min, which far exceeds the industry’s conventional detection threshold of 0.02℃/min. It can sensitively capture subtle temperature changes during battery thermal runaway, and accurately determine key thermodynamic parameters such as the self-heating reaction starting temperature (Tonset), thermal runaway starting temperature (TTR), thermal runaway peak temperature (Tmax), and maximum temperature rise rate (dT/dt) max.
The instrument also meets the “adiabatic temperature rise characteristics” experimental requirements in GB/T 36276-2023 “Lithium-ion Batteries for Electric Energy Storage”, providing strong data support for battery safety performance evaluation, thermal management development, and thermal runaway prevention and control research.
Experimental Procedure
Sample Preparation
The experimental samples include NCM ternary liquid electrolyte lithium-ion batteries with different nickel contents and NCM622 semi-solid lithium metal batteries. Among them, NCM liquid electrolyte batteries can be classified into medium-nickel (NCM532/graphite, NCM622/graphite), high-nickel (NCM811/graphite), and ultra-high-nickel (NCM90505/graphite) batteries based on the differences in nickel content.
Instrument Calibration
- Using the temperature difference baseline mode, the instrument is calibrated with a standard aluminum block of the same size as the battery sample.
- The self-heating rate of the standard aluminum block is measured in HWS mode. Only when the temperature rise rate of the aluminum block at each step during the search period does not exceed ±0.003°C/min can the instrument’s adiabatic performance meet the requirements for subsequent battery thermal runaway testing.
Experimental Operation
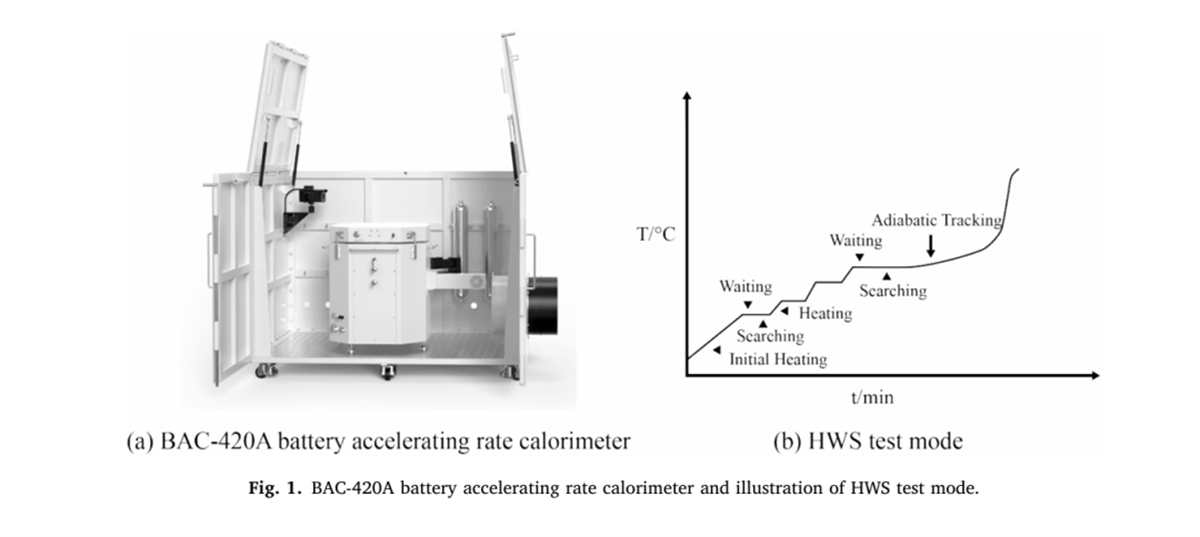
Place the battery inside the furnace chamber of the battery adiabatic calorimeter and conduct an adiabatic thermal runaway test in HWS mode. The experiment consists of a stepwise temperature rise phase and an adiabatic tracking phase. At the start of the experiment, the battery first undergoes a heating-waiting-searching stepwise temperature rise mode until the sample’s temperature rise rate exceeds the 0.02°C/min detection threshold during the stepwise search phase, at which point it enters the adiabatic tracking phase, where the furnace temperature tracks the sample temperature until the battery undergoes thermal runaway.
Experimental Results
Analysis of Thermal Runaway Characteristic Parameters
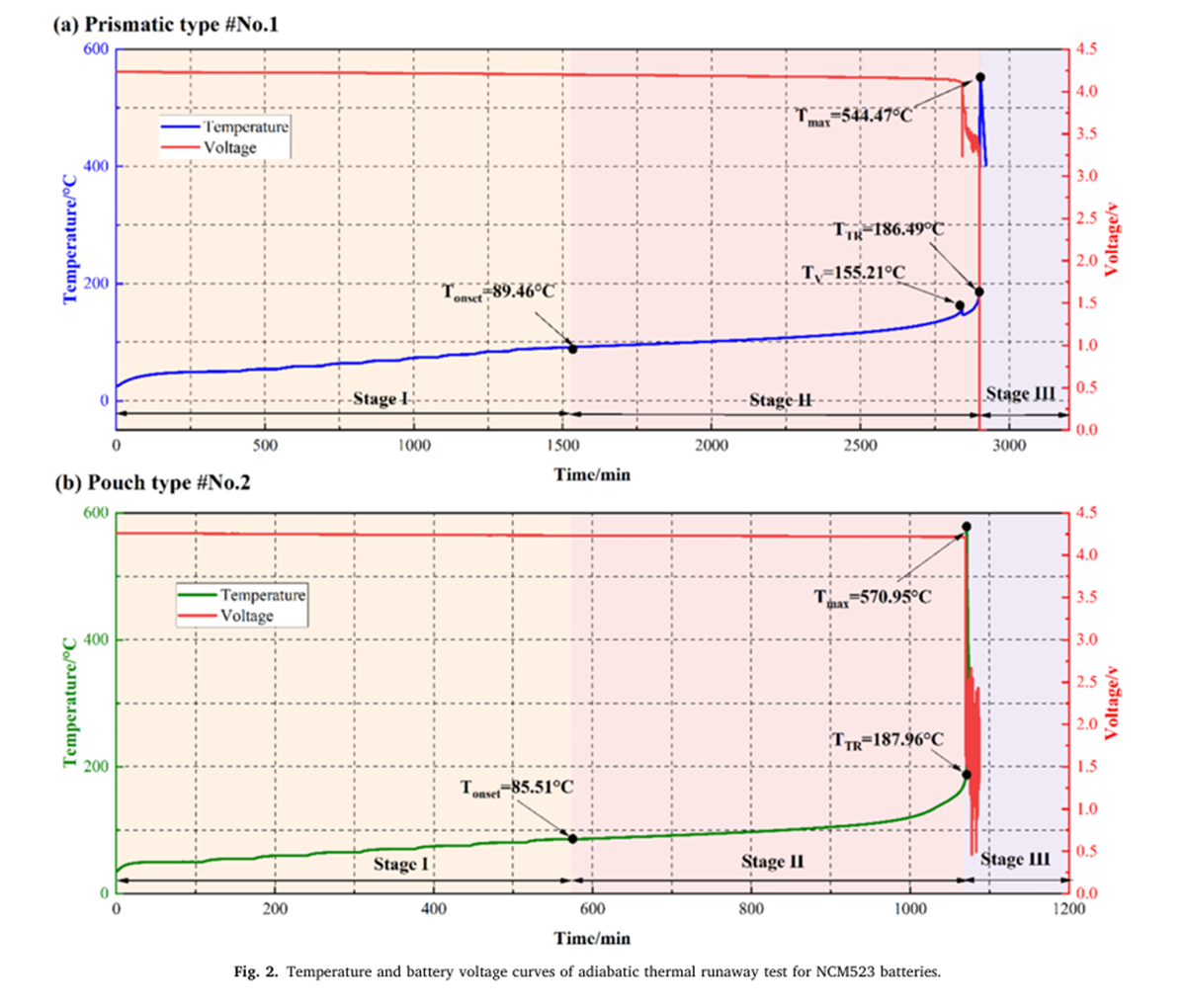
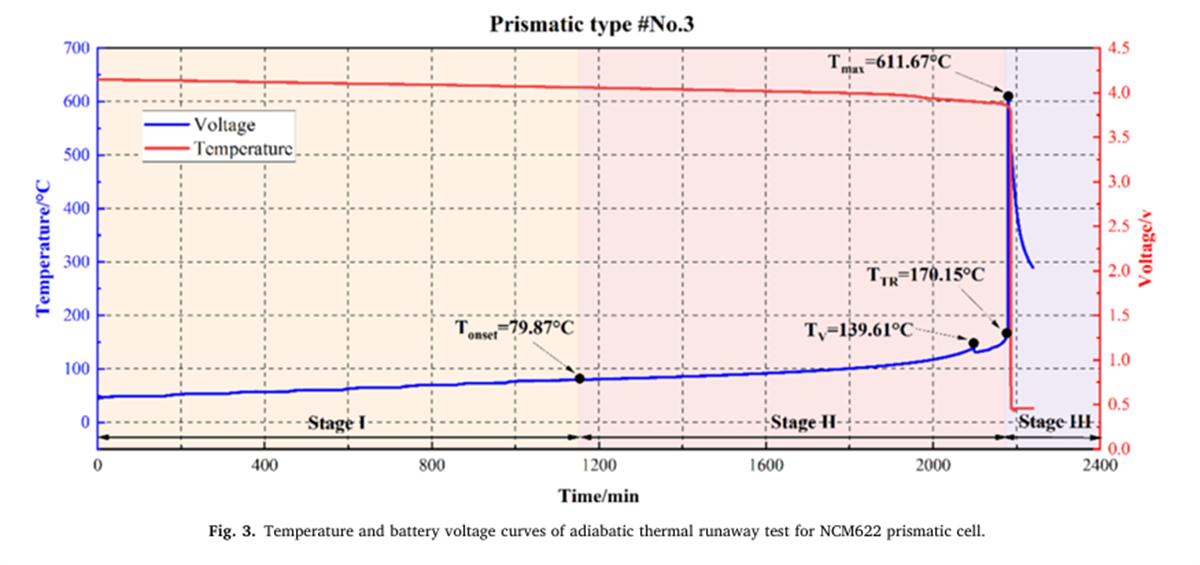
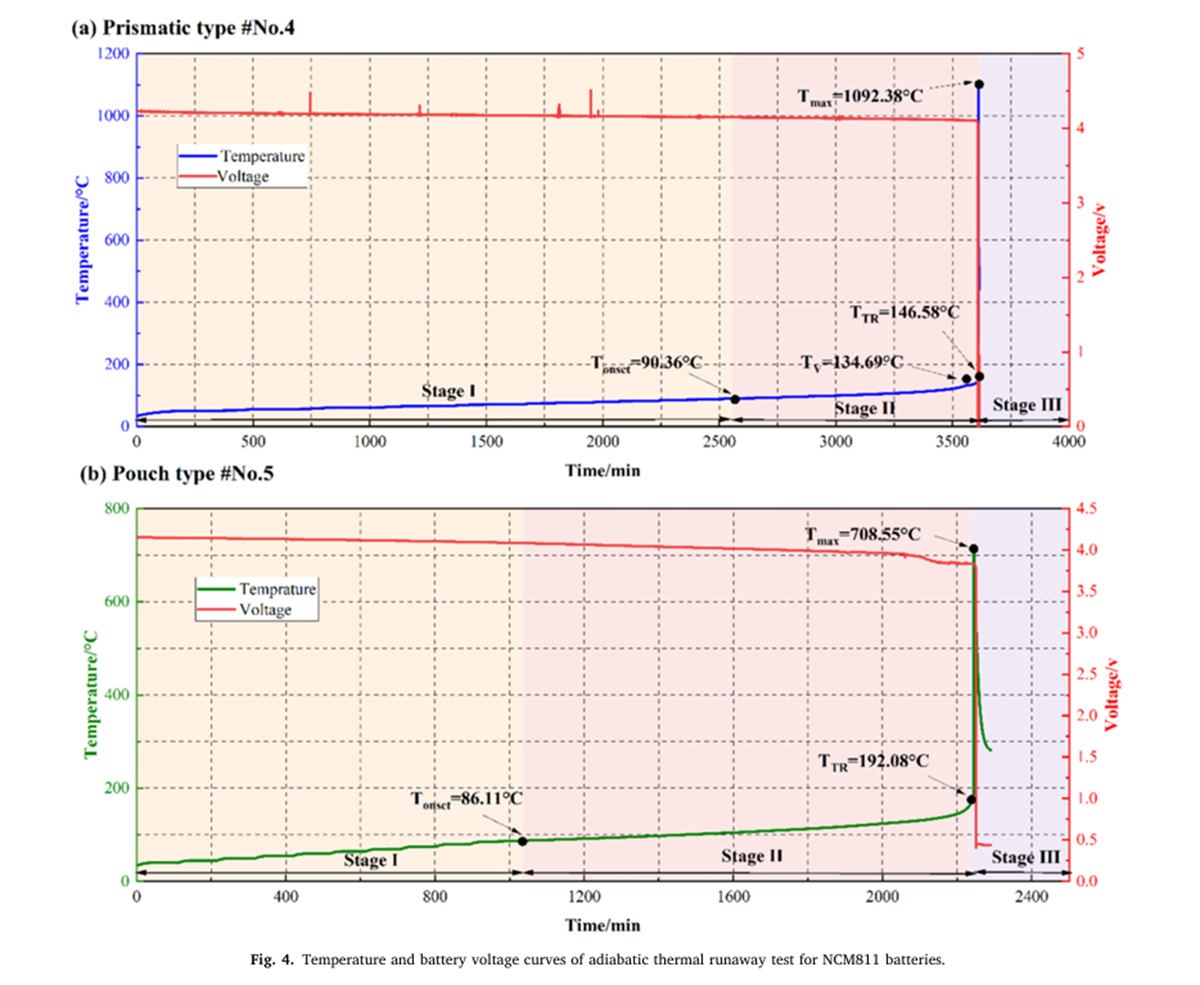
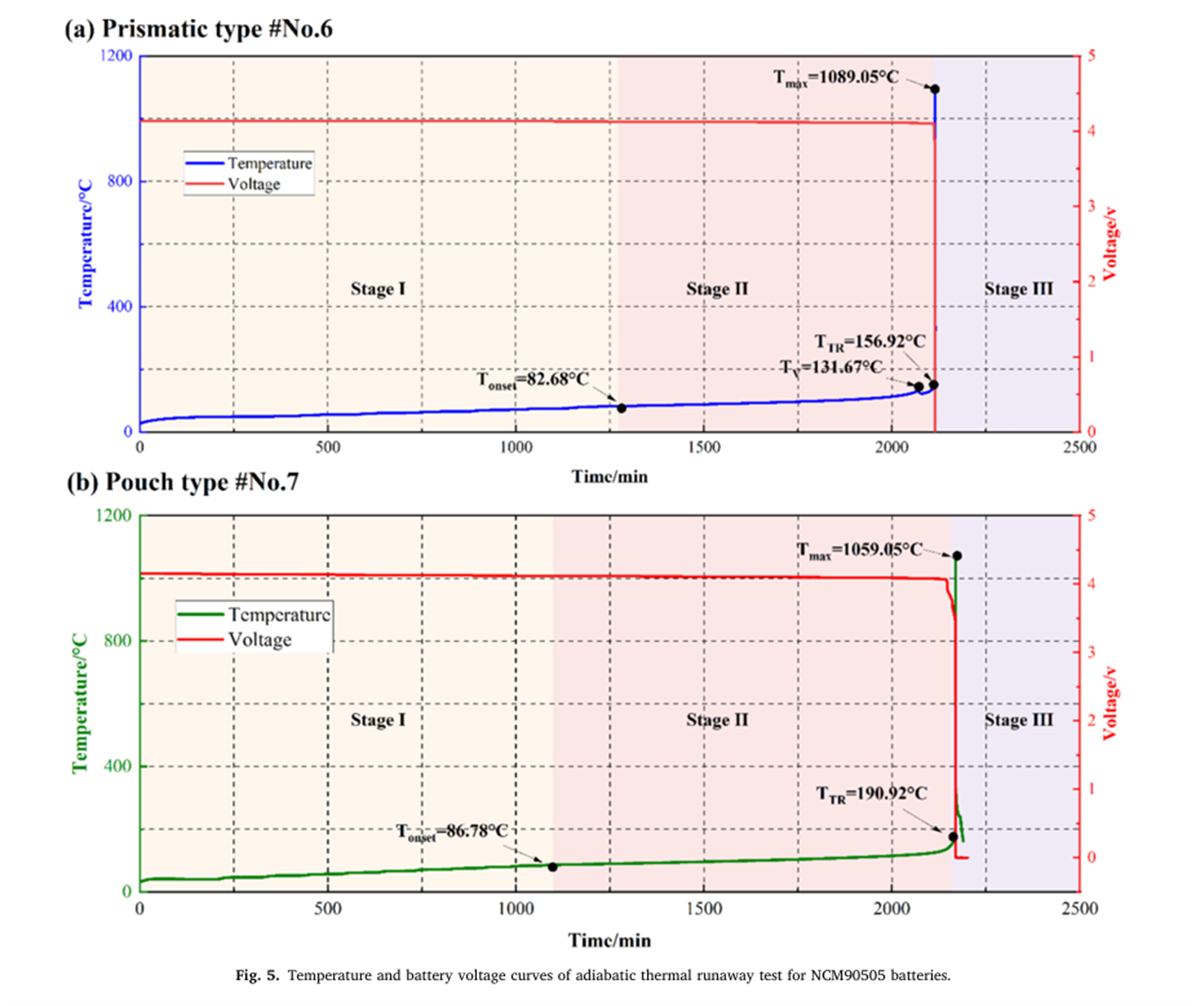
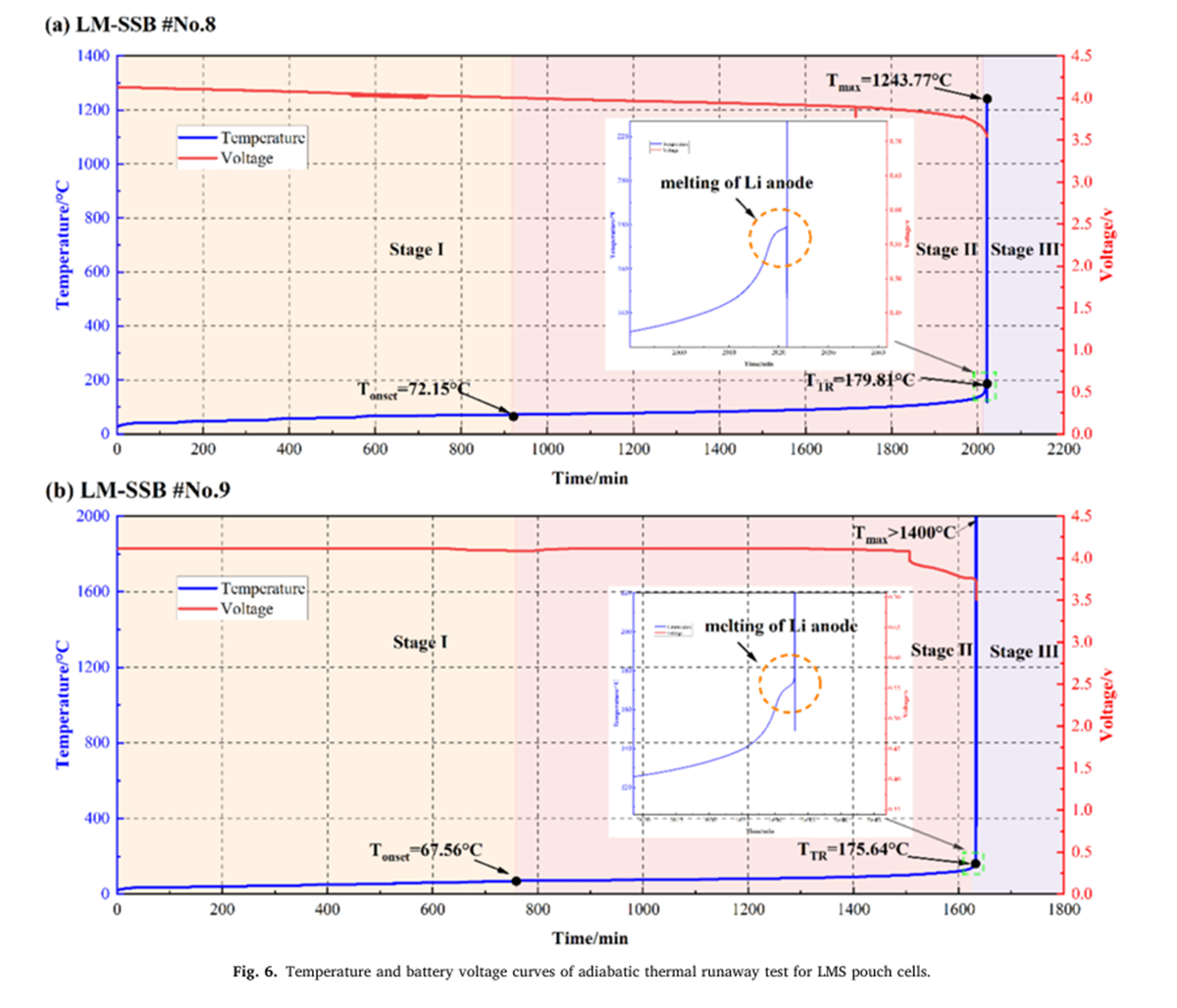
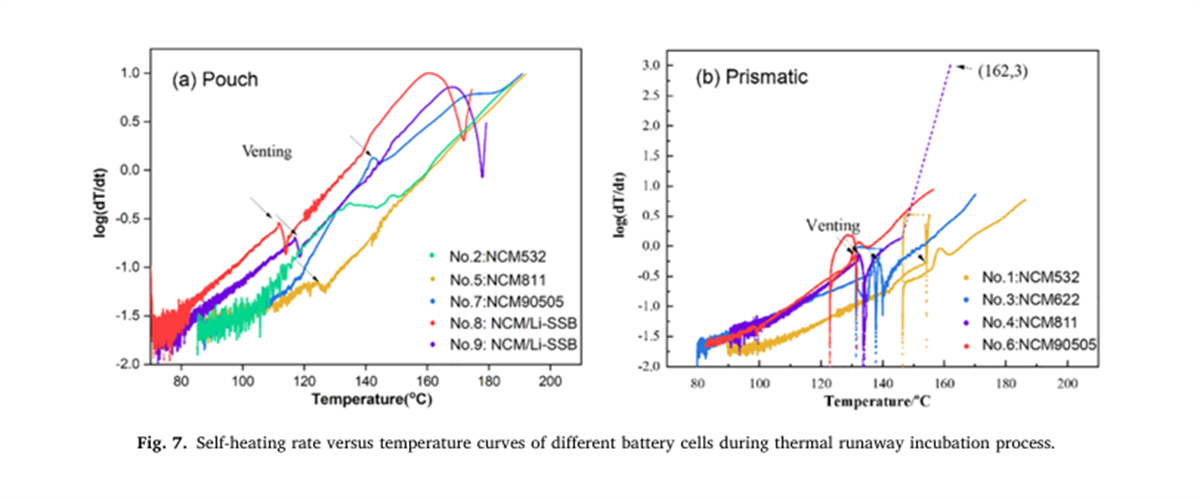
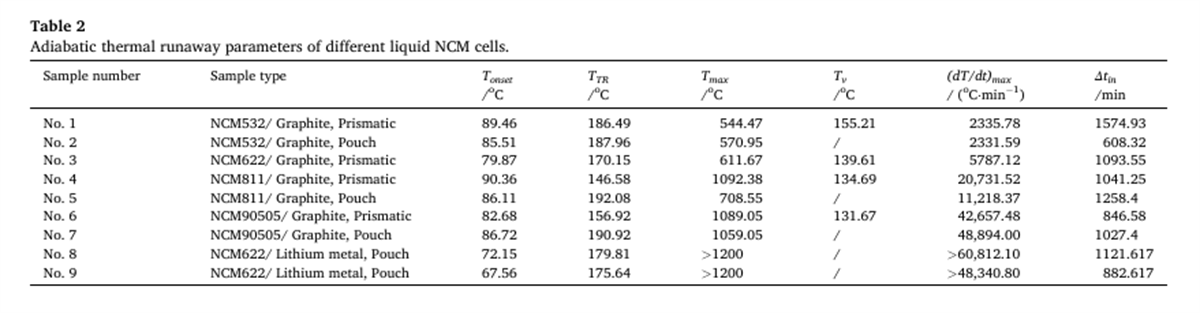
The thermal runaway characteristic parameters of nine different batteries were determined using the BAC-420A large adiabatic battery calorimeter, with the results shown in Figures 2 to 7 and Table 2.
- The Tonset of different NCM liquid lithium-ion batteries generally falls within the range of 80-90°C, indicating that the decomposition mechanism of the SEI film in liquid lithium-ion batteries has a relatively low correlation with the cathode material. As the proportion of nickel in the system increases, the incubation time and duration of thermal runaway shorten, while the energy released per Ah, the rate of energy release, and the mass loss rate during thermal runaway all increase, indicating that the severity of thermal runaway increases with the increase in battery energy density.
- The thermal runaway process of prismatic and pouch lithium-ion batteries within the same system shows differences. The TTR of prismatic batteries decreases with increasing nickel content, gradually reducing from 186.49°C for NCM532 to 156.92°C for NCM90505. In contrast, the TTR of pouch batteries remains around 190°C. Additionally, pouch batteries experience an induction period before rapid temperature rise after reaching TTR.
- The Tonset of lithium metal semi-solid batteries is below 75°C, and Tmax exceeds 1420°C. These batteries have the shortest thermal runaway duration and a mass loss rate close to 100% after thermal runaway. This indicates that compared to high-nickel and ultra-high-nickel NCM ternary lithium-ion batteries, lithium metal semi-solid batteries have lower thermal stability, more severe thermal runaway, and greater potential thermal hazards.
Application of a Two-Step Self-Catalytic Reaction Model
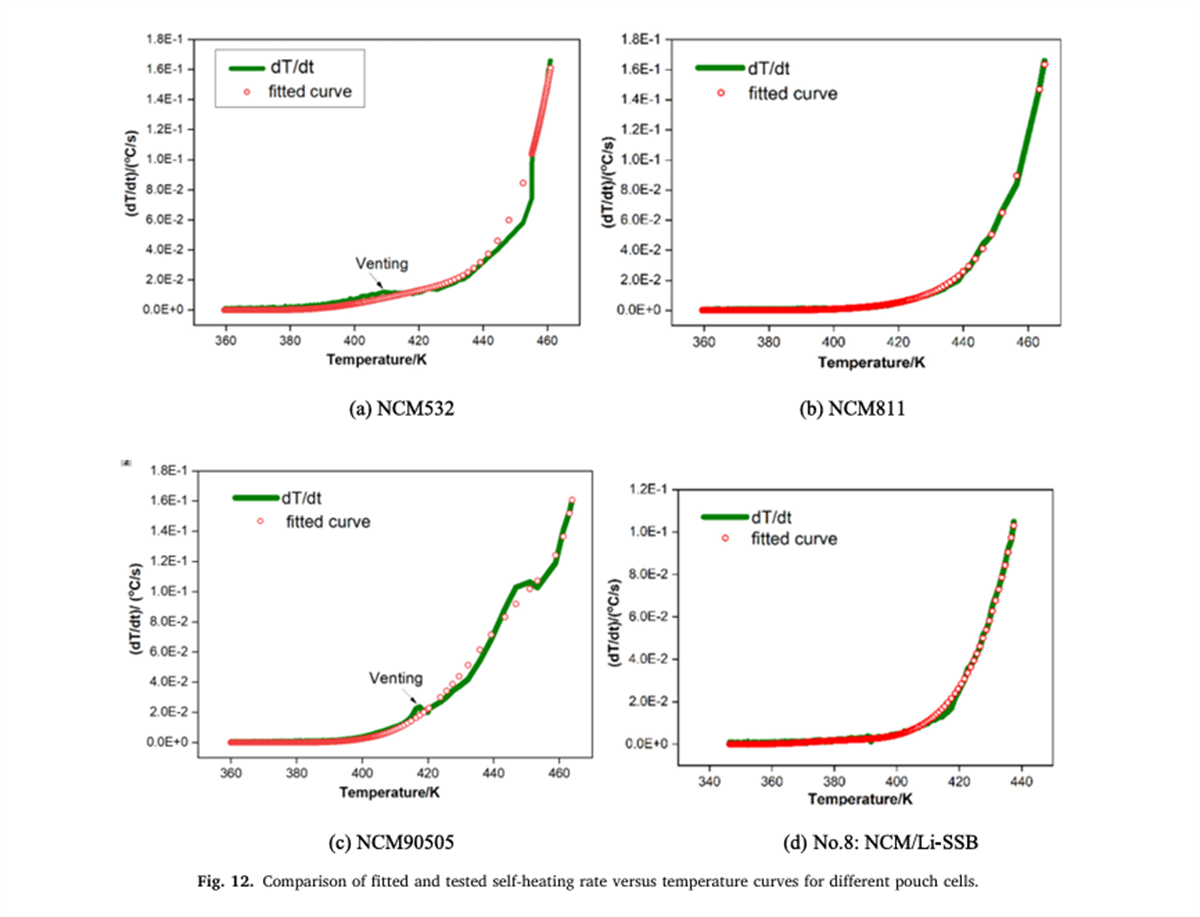
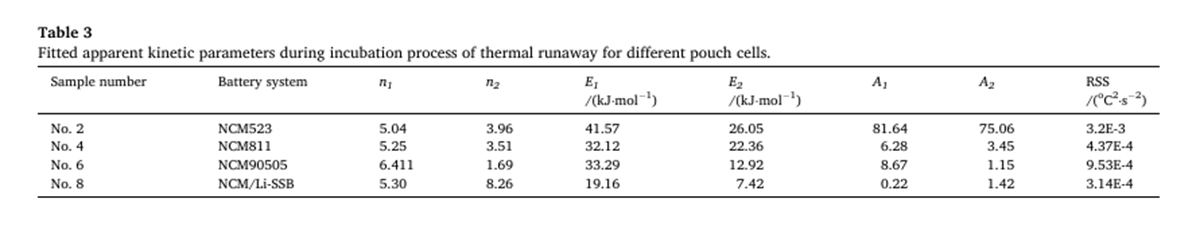
Based on the key thermal runaway parameters measured by the BAC-420A, this study pioneers the application of a two-step self-catalytic reaction model for the apparent kinetic analysis of the battery self-heating process. The results show that the model effectively simulates the apparent thermokinetic process during the incubation stage of thermal runaway and reveals the rule that the activation energy of the reaction during the incubation stage decreases with the increase in energy density.
Conclusion
This study employs the BAC-420A large battery adiabatic calorimeter to conduct an in-depth investigation into the thermal runaway behavior of high-energy-density batteries. It reveals the key characteristics and patterns of thermal runaway in relation to nickel content and battery packaging forms. These findings not only provide references for optimizing the thermal management and safety early warning design of long-range batteries but also offer significant insights for future battery material selection and structural design. As the new energy vehicle industry continues to evolve, research into battery thermal safety will persist, driving technological advancements and sustainable industrial development.








































