Comparison of Isothermal Calorimeter and Battery Adiabatic Calorimeter for Lithium Battery Charge/Discharge Heat Production Measurement

Overview – Isothermal Calorimeter
The utilization of battery Isothermal Calorimeter and large battery adiabatic calorimeters, such as those manufactured by Zeal Instruments, offers valuable insights into battery heat generation behavior. Through careful experimentation and analysis of results, researchers can improve our understanding of lithium-ion battery thermal characteristics and develop effective strategies for thermal management. As battery technology continues to evolve, these insights will be crucial for ensuring the safety, reliability, and performance of lithium-ion batteries in various applications.
Introduction
Accurate measurement of battery heat generation provides valuable insights for thermal management design. Heat generation data helps engineers optimize cooling systems to prevent overheating, which can degrade battery performance and even lead to safety hazards such as thermal runaway. Therefore, precise measurements of heat generation under different operating conditions are essential for developing effective thermal management strategies.
Two primary instruments used for measuring battery heat generation are isothermal calorimeters and adiabatic accelerating calorimeters. These instruments allow researchers to study heat generation in batteries under controlled conditions.
The isothermal calorimeter maintains a constant temperature throughout the battery test. This enables researchers to measure the heat generated by the battery accurately without temperature fluctuations affecting the results. By testing batteries at different temperatures, researchers can assess how temperature variations impact heat generation and, consequently , battery performance.
The adiabatic accelerating calorimeter simulates extreme conditions by preventing heat exchange with the surroundings (adiabatic conditions). This allows researchers to study the maximum heat generation potential of batteries and assess the risks associated with thermal runaway under worst-case scenarios. Understanding the maximum heat generation capability of batteries is crucial for designing safety mechanisms to prevent catastrophic failures.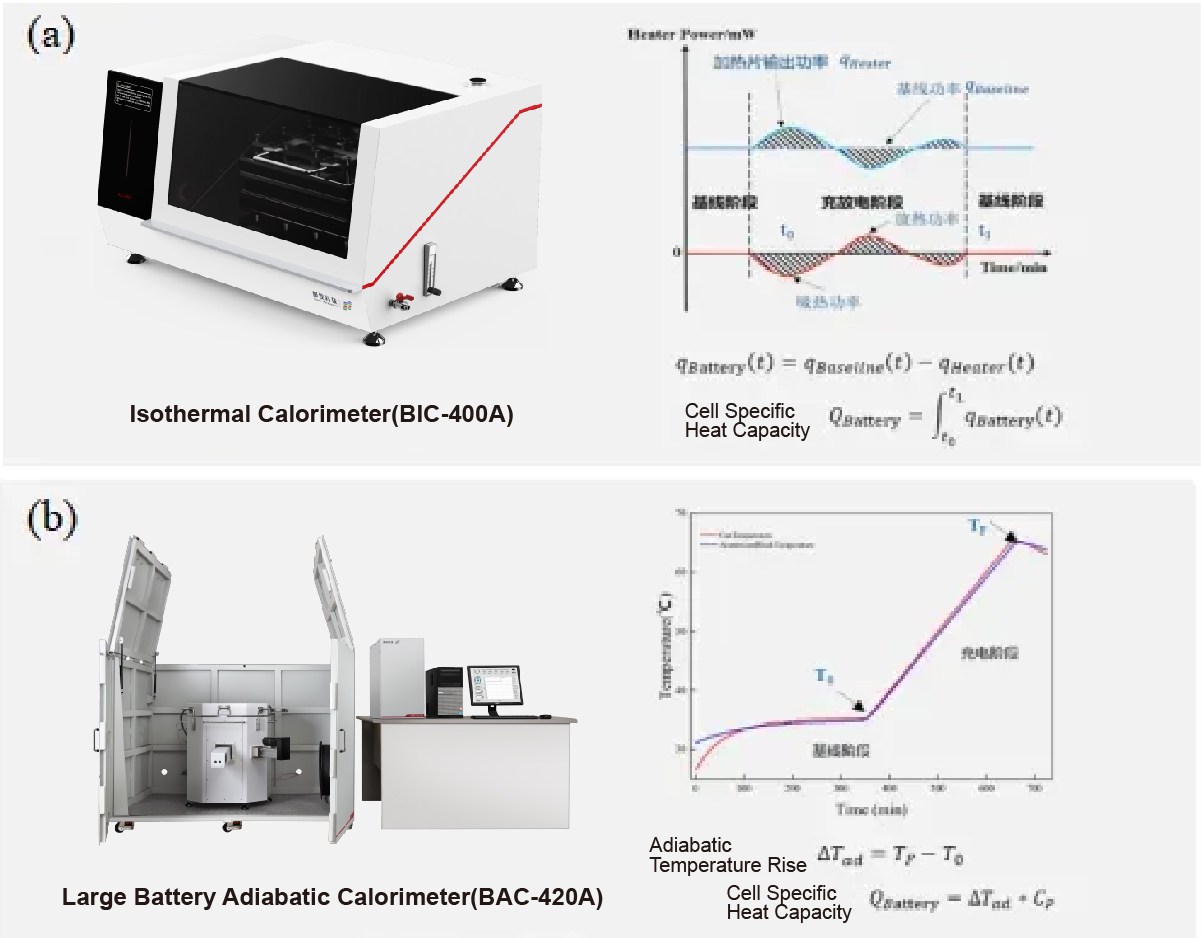
Fig. 1 Detection principle of (a) isothermal calorimeter and (b) adiabatic accelerated calorimeter
Experimental Setup
Preparation of Samples
For the study, 18650 batteries (NCM, 2000mAh) and prismatic batteries (LFP, 50Ah) were used. These batteries represent common types found in various applications. Additionally, aluminum blocks were used to ensure uniform heat distribution during the experiments.
Experimental Conditions
In the adiabatic accelerating calorimeter, the initial temperature was set at 30°C to simulate realistic operating conditions. In contrast, the isothermal calorimeter tests were conducted at temperatures of 30°C and 50°C to evaluate the effects of temperature on heat generation.
Results
Battery Charge and Discharge Mode
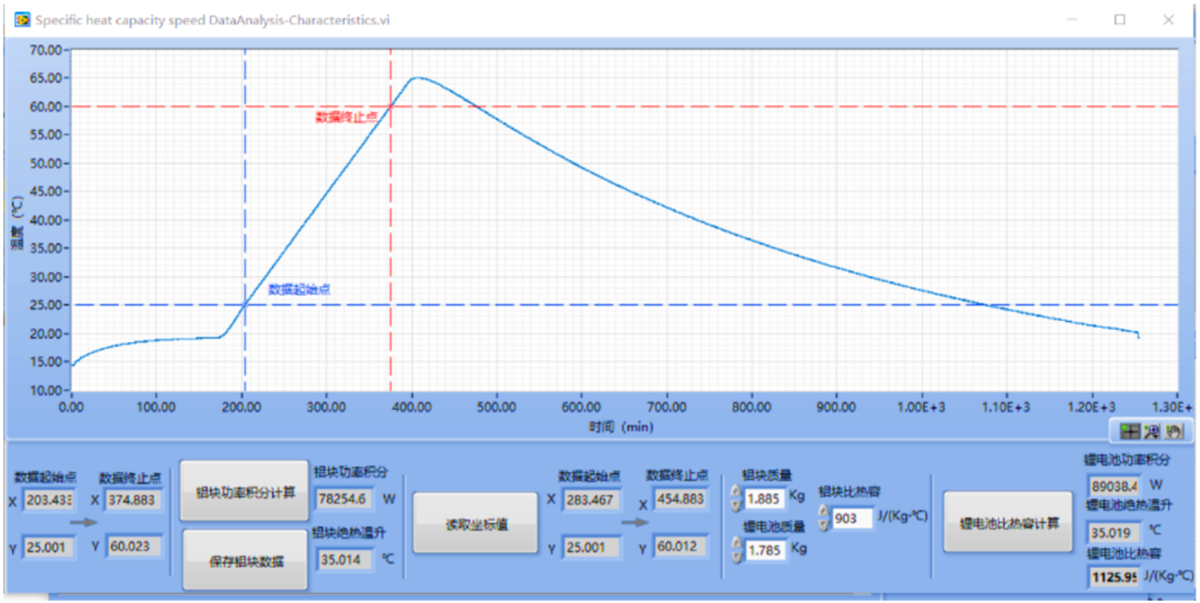
Fig. 2 Measurement results of cell specific heat capacity
Heat Generation Results for 18650 Batteries
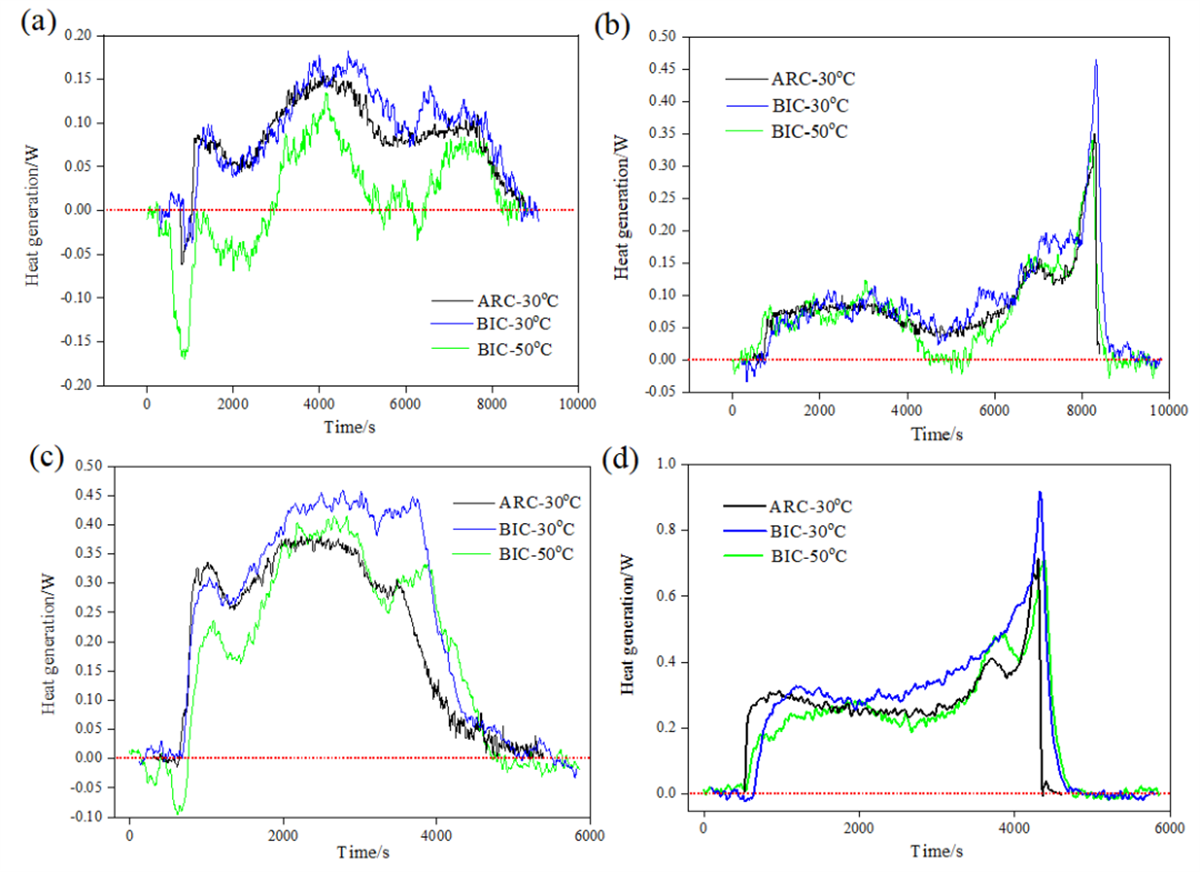
Fig. 3 Comparison of the heat production power measurement results of 18650 cells at (a,b) 0.5C and (c,d) 1C multiplicity for (a,c) charging and (b,d) discharging of the cell
Comparative analysis of heat generation power measurements at 0.5C and 1C rates revealed consistent trends between the isothermal and adiabatic methods.
Despite minor discrepancies, both methods provided valuable insights into the heat generation behavior of 18650 batteries. However, the isothermal calorimeter exhibited a slight lag in heat flow measurement, attributed to its complex sample handling procedures.
Measurement results of charging and discharging heat production under the starting temperature of 30°C are shown in Table 1, and the isothermal calorimetric value is higher than the adiabatic one under four working steps. the adiabatic temperature rise of the battery under 0.5C and 1C is about 15°C and 30°C respectively, and the temperature rise is conducive to lowering the internal resistance of the battery polarization and reducing the heat production of the battery in this range. It can also be seen through Fig. 3 that the power Curves determined by adiabatic method are all between the isothermal calorimetric curves determined under the two temperature conditions of 30°C and 50°C.
Table 1 18650 battery charge/discharge heat production statistics
|
Heat Test condition production J |
0.5C Charge |
0.5C discharge |
1C Charge |
1C discharge |
|
Adiabatic heat |
675.73 |
690.21 |
1033.27 |
1123.23 |
|
Isothermal heat |
772.46 |
833.08 |
1323.23 |
1408.05 |
Heat Generation Results for Prismatic Batteries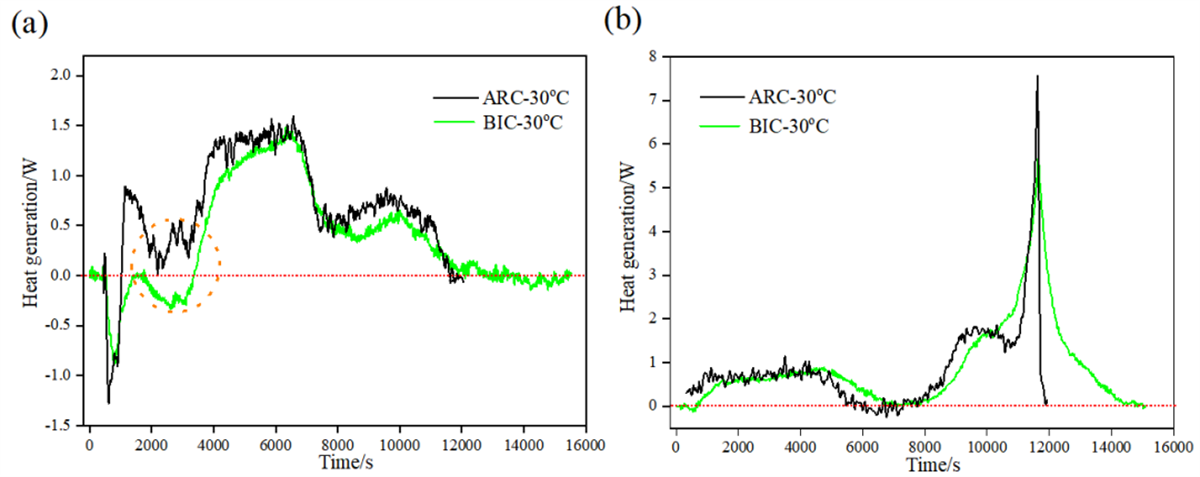 Fig. 4 Comparison of heat production power measurements of square battery during (a) charging and (b) discharging at 0.33C multiplication rate
Fig. 4 Comparison of heat production power measurements of square battery during (a) charging and (b) discharging at 0.33C multiplication rate
Table 2 Comparison of isothermal and adiabatic measurements of heat production at 0.33C multiplication rate
|
Heat Test condition production J |
0.5C Charge |
0.5C discharge |
|
Adiabatic heat |
6291.79 |
11420.11 |
|
Isothermal heat |
5358.45 |
13170.25 |
The study also investigated heat generation during the charge and discharge processes of prismatic batteries, focusing on a lower charge rate of 0.33C. Results indicated higher heat generation measured by the adiabatic method, particularly in cases where pronounced endothermic characteristics were observed during the initial stages of charging. This suggests that the adiabatic method may offer a more accurate assessment of heat generation under specific conditions.
Conclusion
Both the isothermal and adiabatic calorimeters proved effective in measuring battery heat generation. However, differences were observed due to variations in temperature control methods. The adiabatic method typically yielded lower heat generation results compared to the isothermal method when battery temperature remained within the normal operating range .
While the adiabatic method generally provides reliable results, it may yield higher heat generation measurements under low-rate charging conditions with significant endothermic characteristics. Engineers and researchers must consider these differences when selecting the appropriate testing method based on the specific requirements of their studies or applications .
Reference
[1] Noboru Sato. [J]. Thermal behavior analysis of lithium-ion batteries for electric and hybrid vehicles. Journal of Power Sources, 99 (2001):70-77.