Determination of Calorific Value of Classic Puffed Foods by Oxygen Bomb Calorimeter
Preview
This article uses the oxygen bomb calorimeter(ATC 300A) to test the combustion heat values of four types of puffed food (potato chips, rice crackers, small steamed buns, and crispy noodles). The difference between the test results and the energy values on their packaging is between 0.16 and 0.53 kcal/g, with RSD (Relative Standard Deviation) all within 0.2%.
Calories, a measure of heat, are integral to nutritional assessment and fitness guidance, alongside energy values denoted in joules on food labels. These values indicate the heat emitted during food oxidation, with a conversion factor of 1 cal = 4.1868 J. The question arises: How does the heat provided by food differ from that released upon its complete combustion?
Introduction
Digestion and absorption within the human body are intricate processes. Certain food components, such as nitrogen in proteins, are indigestible and remain in metabolic byproducts like urea, uric acid, and creatinine, retaining a portion of energy. Despite the human body’s oxidation differing from that of an oxygen bomb calorimeter, the total heat released from food’s complete oxidation remains consistent. To ascertain the physiological heat value of food, metabolic adjustments can be applied to the combustion test results from an oxygen bomb calorimeter. By measuring the combustion heat of food and the heat of excreta, one can ascertain the effective heat value of a particular food item, disregarding complex factors like basal metabolism.
Energy values listed on nutritional information tables are calculated as the sum of the products of the energy coefficients for the three primary nutrients (fat at 37 kJ/g, carbohydrates at 17 kJ/g, and protein, after metabolic correction, at 17 kJ/g) and their respective quantities. This study employs the ATC 300A automatic oxygen bomb calorimeter to determine the combustion heat values of four types of puffed foods, comparing these to the energy values listed on food labels. Additionally, energy values are calculated without metabolic correction for protein, using an energy coefficient of 22 kJ/g. The study reveals that while the overall deviation due to metabolic correction is minimal, variations in combustion heat values among different food samples are observed. These discrepancies may stem not only from protein content but also from the diverse origins of identical nutrients; for instance, fats in beef and milk have distinct combustion heat values, yet they share the same energy coefficient in nutritional categorization.

Figure 1: Display of Test Samples

Figure 2: ATC 300A Oxygen Bomb Calorimeter
Experimental Methods
Experimental Conditions
- Testing Instrument: Automatic Oxygen Bomb Calorimeter ATC 300A
- Testing Method: GB/T 213-2008
- Ambient Temperature: 24.4~26.3℃
- Experimental Samples: Potato chips, rice crackers, small steamed buns, and crispy noodles
Testing Procedure
- Power on the Automatic Oxygen Bomb Calorimeter ATC 300A.
- Step 1: Weigh a certain amount of the sample in the sample pan, connect the ignition wire to the sample with cotton thread, and secure it.
- Step 2: Install the oxygen bomb, set the experimental parameters, and enter the sample mass.
- Step 3: Initiate the experiment; once the testing environment is ready, the instrument will automatically conduct the test.
- Step 4: After the experiment concludes, remove the oxygen bomb and clean it.
- Step 5: Repeat the test for three sets and record the experimental data.
Experimental Results
Prior to commencing the experiments, each sample was subjected to crushing and pelletizing to ensure uniformity and consistency of the test samples, as depicted in Figure 3. During the pelletizing process, it is crucial to control the pressure applied; for instance, due to the high oil content in potato chips, excessive pressure could cause oil to seep out, thereby affecting the test outcomes.

Figure 3: Sample Pre-treatment (a) Crushed Samples (b) Display of Small Steamed Bun Pellets (c) Pelletized Samples (d) Sample Loading
Each sample type—potato chips, small steamed buns, rice crackers, and crispy noodles—was subjected to three repeated tests. The results are summarized in the table below, showing good test repeatability with Relative Standard Deviations (RSD) all within 0.2%.
Table 1: Summary of Combustion Heat Test Results
|
Combustion Heat (J/g) |
Potato Chips |
Small Steamed Buns |
Rice Crackers |
Crispy Noodles |
| 1 | 23935.0 | 16548.9 | 21535.5 | 22750.7 |
| 2 | 23925.7 | 16558.1 | 21505.3 | 22766.8 |
| 3 | 23995.1 | 16544.9 | 21505.2 | 22771.6 |
|
Average Combustion Heat |
23951.9 | 16550.6 | 21515.3 | 22763.0 |
|
Packaging Energy Value |
22666.7 | 15870.0 | 20620.0 | 20550.0 |
|
Metabolic Correction-Free Energy Value |
22967.6 | 16017.3 | 20860.7 | 21018.1 |
| RSD(%) | 0.157 | 0.041 | 0.081 | 0.078 |
The average combustion heat values, when compared to the energy values listed on the nutritional information on the packaging (as depicted in Figure 4, with a protein energy coefficient of 17 kJ/g), show a difference ranging from 680.6 to 2213.0 J/g. Without considering the metabolic correction for protein (with an energy coefficient of 22 kJ/g), the difference ranges from 533.3 to 1745.0 J/g.
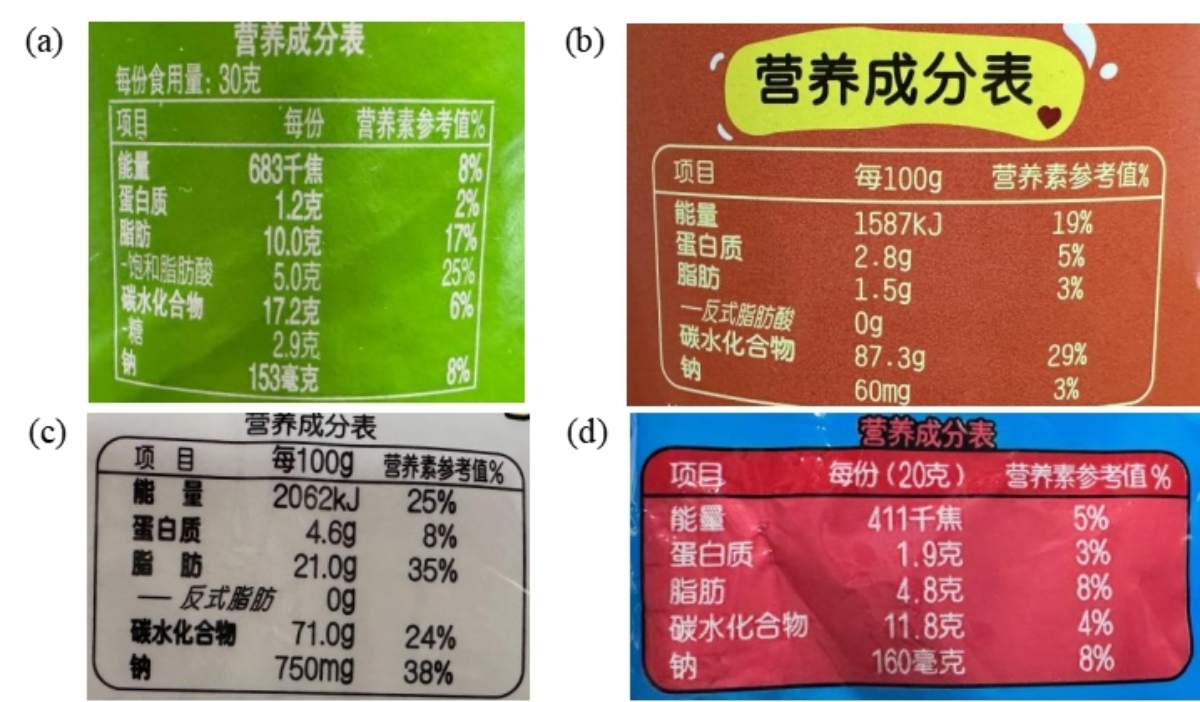
Figure 4: Nutritional Information on Packaging (a) Potato Chips (b) Small Steamed Buns (c) Rice Crackers (d) Crispy Noodles
Given that the samples selected for this study are puffed foods, which are primarily composed of fats and carbohydrates with relatively low protein content, the impact of metabolic correction on the test results is relatively minor. Instead, it is more likely that the energy parameters for the same nutrients from different sources introduce some variation.
Based on the test results, the combustion heat value can to some extent represent the “calories” we can obtain from food. In addition to human metabolism, using the same energy parameters for the same nutrients from different sources can also introduce certain errors. Taking the puffed foods tested in this study as an example, the difference between the combustion heat value without considering protein metabolic correction and the packaging energy value is 12.7 to 41.7 kcal (kilocalories) per 100g. For individuals who strictly monitor their “calorie” intake, this impact may need to be taken into consideration.
Conclusion
This study utilized the Automatic Oxygen Bomb Calorimeter ATC 300A to measure the combustion heat values of four types of puffed foods. The results closely align with the energy values listed on their packaging. The discrepancies may encompass adjustments based on nutritional science for the combustion heat values of different nutrients according to human metabolism, as well as variations in energy parameters for the same nutrients from different sources.
Instrument Recommendation
The ATC 300A Automatic Oxygen Bomb Calorimeter complies with standards such as GB 384, GB/T 213, ASTM 4809, and ASTM D240. It offers a testing time of less than 10 minutes (quick method), with a heat capacity fluctuation of ≤0.20%. Highly automated, it can quickly and accurately measure the combustion heat values of various combustibles. For more information on technical highlights, specifications, and application cases, please feel free to contact us.







































