Enhancing Safety in Fine Chemical Processes: Exploring Accelerating Rate Calorimeter
 Background Introduction
Background Introduction
China is a major player in the chemical industry, with a total output value of 13.7 trillion yuan in 2018, accounting for 15.2% of the national GDP and about 40% of the global chemical output, ranking first in the world. To increase industry value -added, after decades of development, the refinement level of the domestic chemical industry has continuously improved, reaching around 50% currently. Fine chemical processes are mostly batch or semi-batch closed production methods, characterized by small scale, diverse varieties, complex processes , and rapid changes. The rapid development of the domestic fine chemical industry poses certain challenges to production safety management. In 2019, a total of 164 chemical accidents occurred nationwide, resulting in 274 deaths. Among them, there were 2 major accidents with 25 deaths and 1 particularly major accident with 78 deaths. For three years consecutive since 2017, there have been more than 2 major accidents annually. The situation of chemical safety production remains very grim, and the risk assessment of reaction safety in fine chemical processes is crucial Learn more about Automatic Reaction Calorimeters.
Reaction Runaway
Chemicals have unique thermal hazards and can undergo reactions under specific conditions, releasing a large amount of heat. The increase in temperature leads to an acceleration in the reaction rate, which in turn generates more heat, further increasing the reaction temperature. This feedback mechanism leads to chemical reaction runaway. Reaction runaway is the main cause of chemical accidents. Chiba-Geigy company compiled factory accidents that occurred between 1971 and 1980, among which 56% of the accidents were caused by reaction runaway or near-runaway.
The essence of runaway is the imbalance between the heat generation rate of the reaction system and the heat transfer rate, resulting in heat accumulation. Under normal process conditions, chemical reactions proceed at controllable temperatures. However, if cooling fails or feed rates are too fast , for example, the heat generated by the reaction cannot be completely removed by the reactor cooling system, causing the reaction temperature to deviate from normal conditions. Once the temperature reaches the decomposition temperature of the materials, secondary reactions are triggered, causing the decomposition of reactants and products, resulting in a sharp increase in temperature and pressure inside the reactor, ultimately leading to material ejection, reactor damage, and even combustion or explosion accidents.
Evaluating the runaway process of chemical reactions requires considering several important parameters:
- Process temperature (T p ): Also the initial temperature when cooling fails;
- Maximum temperature that the runaway system can reach (MTSR): The maximum temperature that the synthetic reaction may reach under adiabatic conditions, considering the maximum accumulation of materials during the reaction process;
- Adiabatic temperature rise (ΔT ad ): Considering adiabatic conditions, all the heat released by the reaction system is used to raise the temperature of the reaction system;
- Time required for the maximum reaction rate of the runaway reaction (TMR ad ): The time required for the runaway reaction to reach the maximum reaction rate under adiabatic conditions, which can be colloquially understood as the time to explosion;
- Temperature at 24 hours when the maximum reaction rate is reached (T D24 ): The temperature corresponding to TMRad equals 24 hours, generally considered as the temperature at which the sample begins to decompose;
- Maximum technical temperature (MTT): The temperature corresponding to the boiling point of the solvent or the maximum allowable pressure of the reaction vessel.
Risk Assessment of Reactions
The former State Administration of Work Safety issued the “Guiding Opinions on Strengthening Safety Risk Assessment of Fine Chemical Reactions” in 2017, which clearly stipulates testing the thermal stability of raw materials, intermediates, and products involved in chemical reactions, conducting thermodynamic and kinetic analysis of chemical reaction processes, evaluating reaction risk levels based on parameters such as reaction heat and adiabatic temperature rise, assessing the possibility of reaction runaway based on parameters such as the time to reach maximum reaction rate, and determining reaction process risk levels and recommendations based on relevant reaction temperature parameters.
Ev aluation of Material Decomposition Heat
|
Class |
Heat of decomposition(J/g) |
Description |
|
1 |
Heat of decomposition<400 |
potential explosion risk |
|
2 |
400<Heat of decomposition<1200 |
High risk of potential explosion |
|
3 |
1200<Heat of decomposition<3000 |
Higher risk of potential explosion |
|
4 |
Heat of decomposition≥3000 |
Highest risk of potential explosion |
Evaluation of Reaction Process Risk
- Severity Assessment
|
Class |
△T ad (K) |
Consequence |
|
1 |
≤50 and no pressure effect |
Loss of material from a single batch |
|
2 |
50<△T ad<200 |
Short-term damage to plant |
|
3 |
200≤△Tad < 400 |
Severe plant damage |
|
4 |
≥400 |
Destructive plant loss |
- Likelihood Assessment
|
Class |
TMR ad (h) |
Consequence |
|
1 |
TMR ad ≥24 |
Rarely occurs |
|
2 |
8<TMR ad<24 |
Occasionally occurs |
|
3 |
1<TMR ad ≤8 |
Likely to occur |
|
4 |
TMR ad ≤1 |
Frequent |
- Matrix Assessment (Risk = Severity × Likelihood)
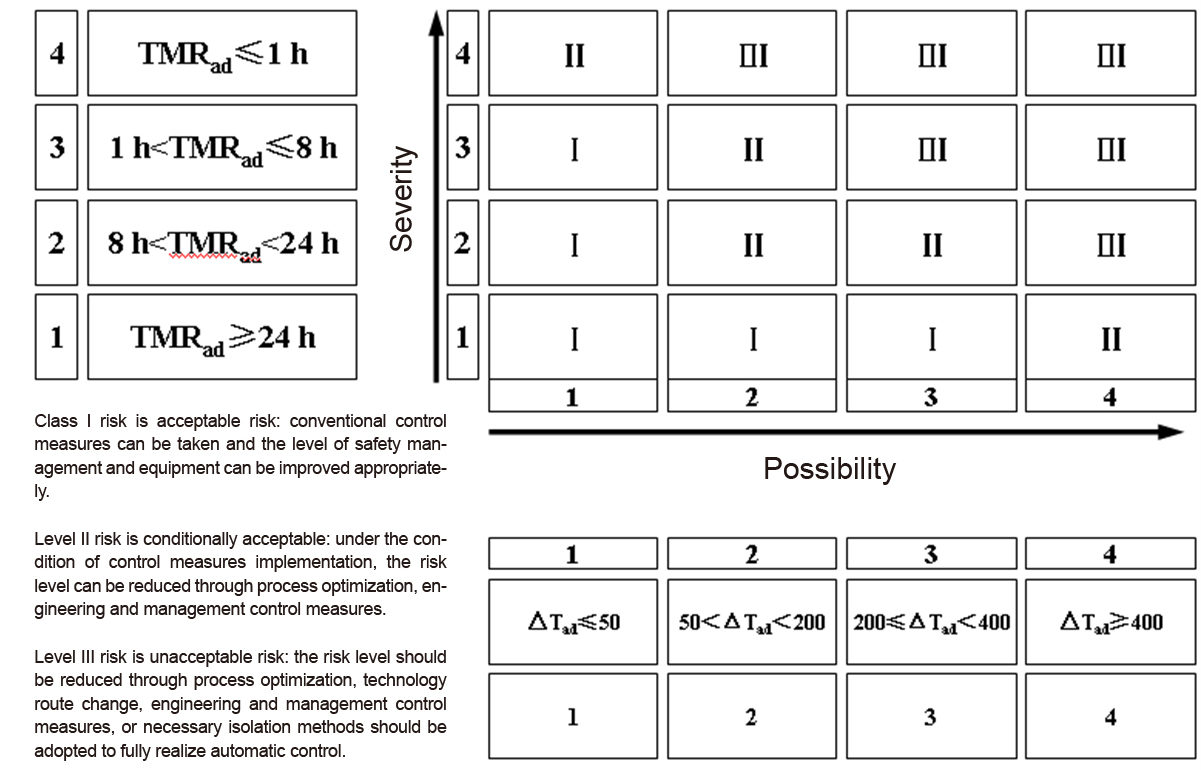
Reaction Process Hazard Assessment
Based on different levels of reaction process risk, different risk control measures need to be established. For processes with a risk level of 3 or above, it is necessary to further obtain parameters such as runaway reaction temperature, relationship between runaway reaction system temperature and pressure , maximum temperature during runaway process, maximum pressure, maximum temperature rise rate, maximum pressure rise rate, and adiabatic temperature rise, and determine corresponding risk control measures.
|
Class |
Temperature |
Consequence |
|
1 |
T p<MTSR<MTT<T D24 |
Reaction risk is low |
|
2 |
T p<MTSR<T D24<MTT |
Potential decomposition risk |
|
3 |
T p≤ MTT<MTSR<T D24 |
washout and decomposition |
|
4 |
T p≤ MTT<T D24<MTSR |
High risk of washout and decomposition, potential explosion risk |
|
5 |
T p<T D24<MTSR<MTT |
High risk of explosion |
Key Instruments for Reaction Risk Assessment
A variety of instruments are required for reaction safety risk assessment, including reaction calorimeters, adiabatic accelerating rate calorimeters, differential scanning calorimeters, flash point testers, explosion limit testers, minimum ignition energy testers, moisture analyzers, liquid chromatographs, gas chromatographs, and other analytical instruments. The key instruments for obtaining the main evaluation indicators are reaction and accelerating rate calorimeters.
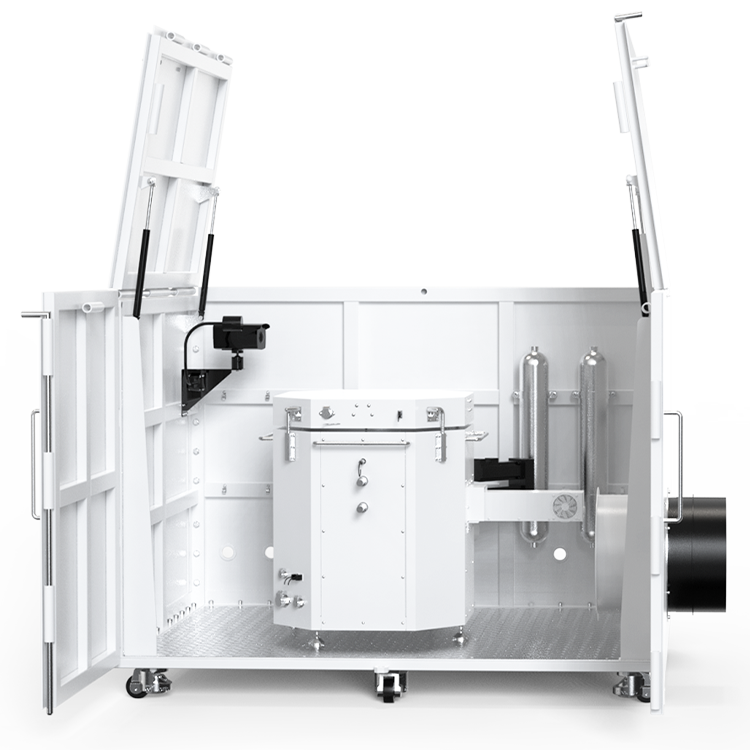
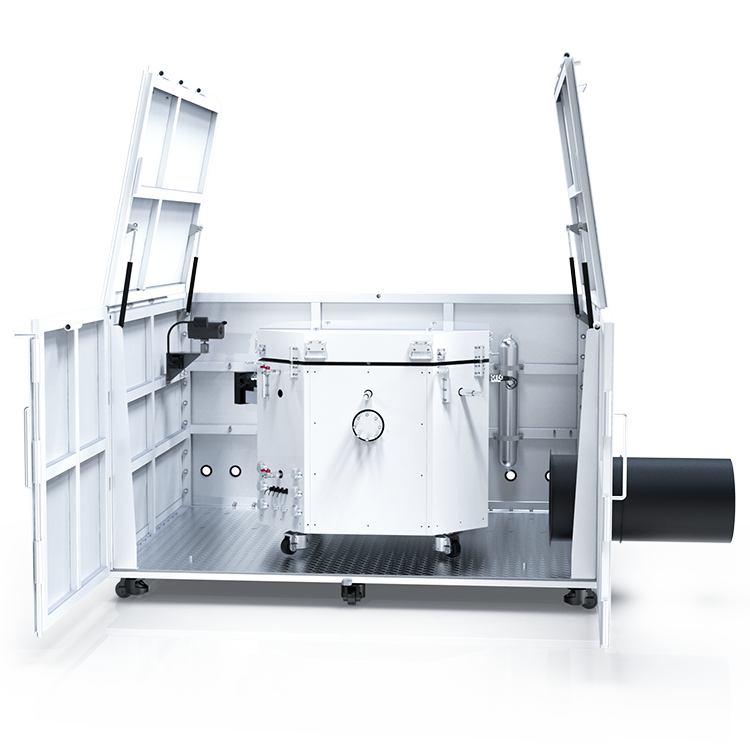
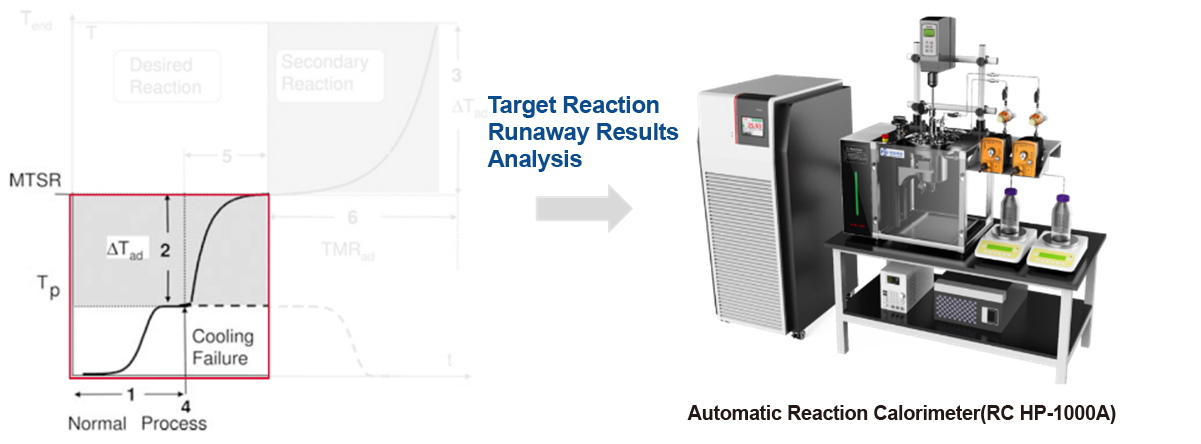
Automatic Reaction Calorimeter
The reaction calorimeter can simulate the specific process of chemical reactions on a liter scale, analyze the runaway results of target reactions, and measure data such as adiabatic temperature rise (ΔT ad ) and the maximum temperature (MTSR) of the runaway system.
 |
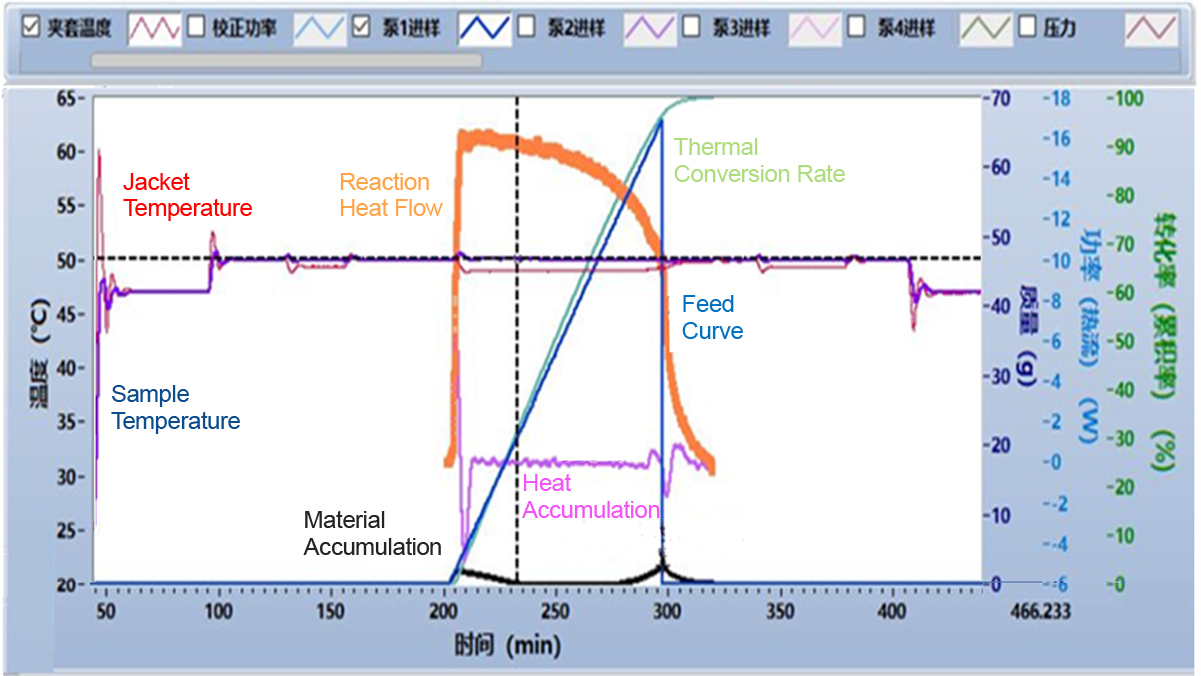 |
The reaction calorimeter software allows for the setup of multi-step experimental processes, with precise temperature control achieved through either oil bath or heater temperature regulation for each step of the reaction. After the experimental process is completed, the software automatically calculates various data including the heat release rate (heat flow) at different time points, material and heat accumulation, heat conversion rate, and MTSR (Maximum Temperature of the Self-Heat Runaway). These data intuitively reflect the heat release characteristics and process changes of the target reaction. The main parameters can be calculated using the following formulas:
(1) Heat release enthalpy:ΔH = ∫Qr/m
Where Qr is the heat release rate (W), and m is the total mass of reactants (g).
(2) Heat conversion rate:a% = Ht/Htotal
Where Ht is the total heat release up to time t (J), and Htotal is the total heat release from the beginning to the end of the reaction (J).
(3) Material accumulation:Xacc = ∫dmr – m * a%
Where dmr is the feed rate (g/s).
(4) Adiabatic temperature rise:ΔTad = ∫dQr/(Cp * mtotal) * Xacc
Where mtotal is the total mass of the reaction system, and Cp is the specific heat capacity of the material.
(5) Maximum Temperature of the Self-Heat Runaway (MTSR):MTSR = Tp + ∫dQr/(Cp * mtotal) * Xacc,max
Where Tp is the process temperature and Xacc, max is the maximum material accumulation.
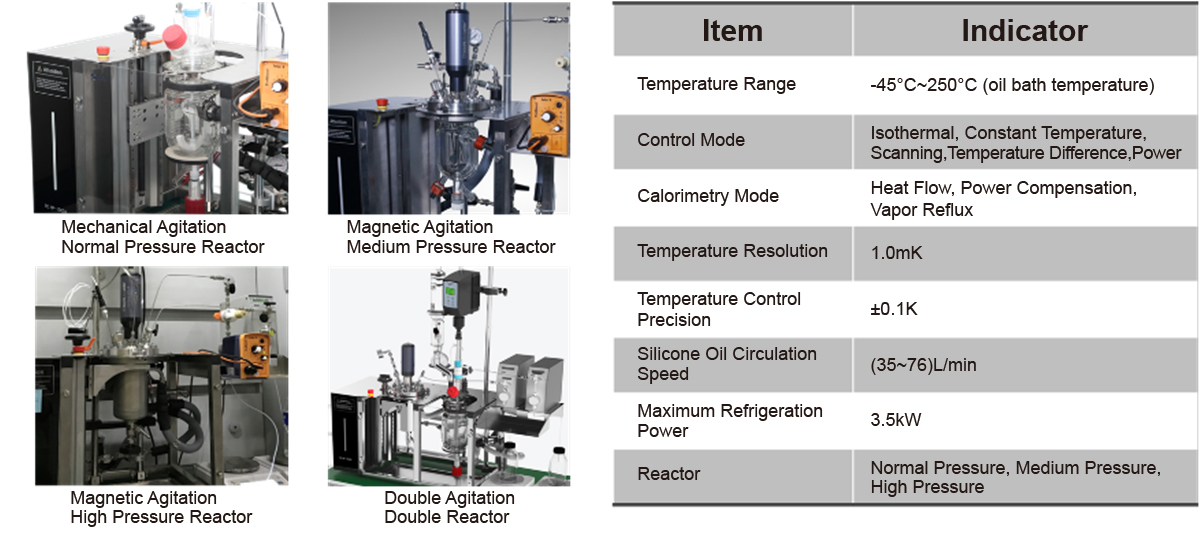
The RC HP 1000A is the first domestically developed automatic reaction calorimeter. Its features include:
- High temperature control accuracy, with temperature control accuracy of ±0.1K over the entire temperature range.
- Multiple calorimetric modes, supporting heat flow, power compensation, and reflux heat flow modes.
- High measurement accuracy, considering heat transfer and heat loss throughout the calorimetric process, and capable of calibrating system heat capacity over the entire temperature and liquid level range.
- Strong customization capability, with flexible component configurations and support for custom software function development.
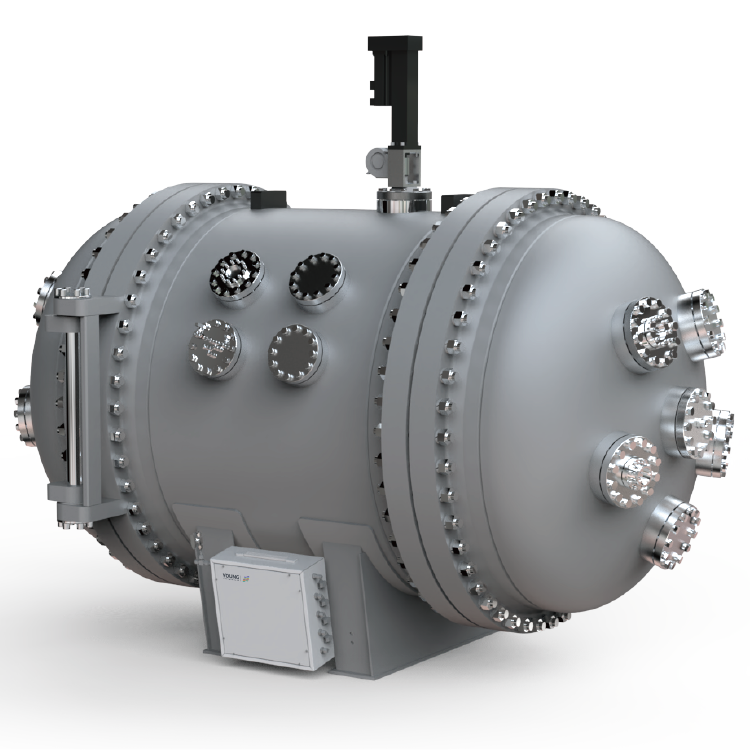
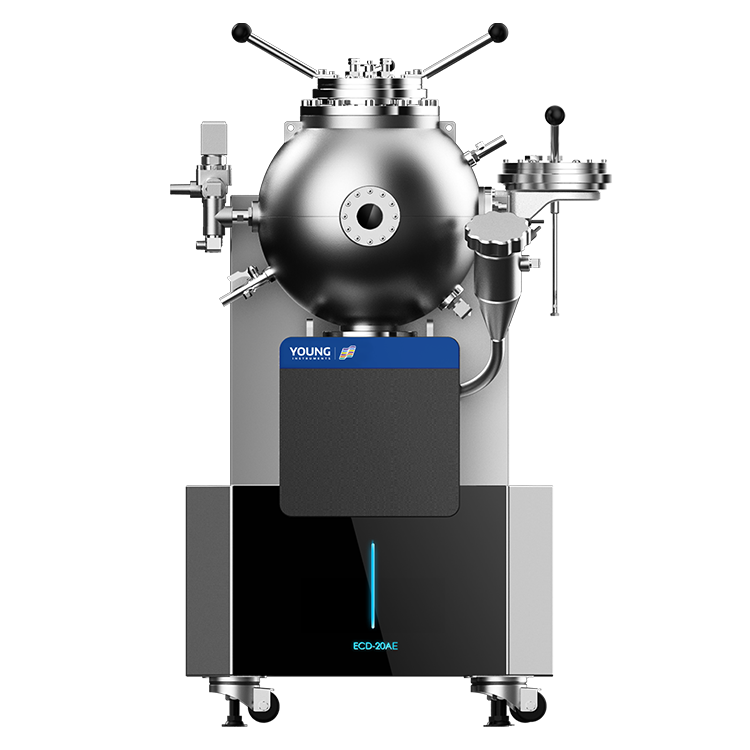
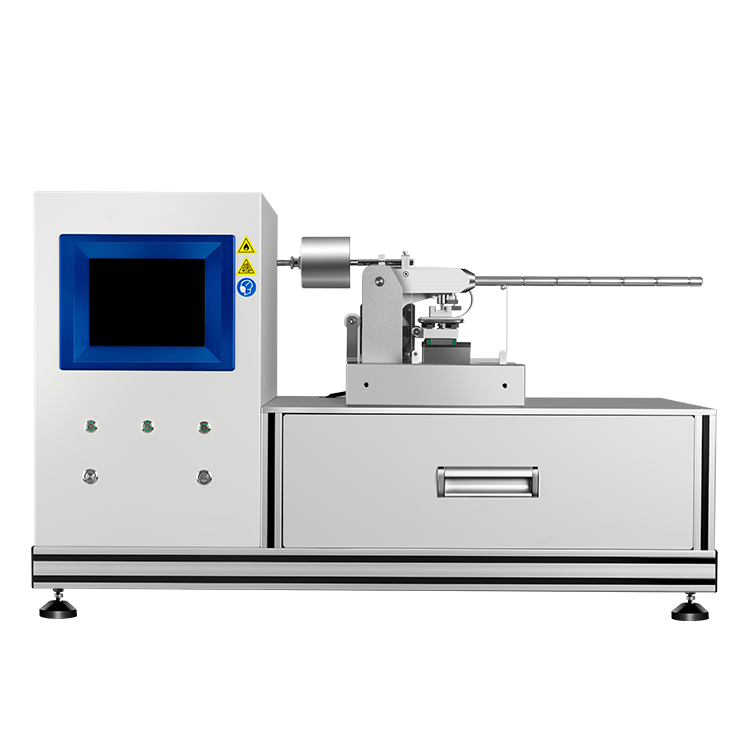
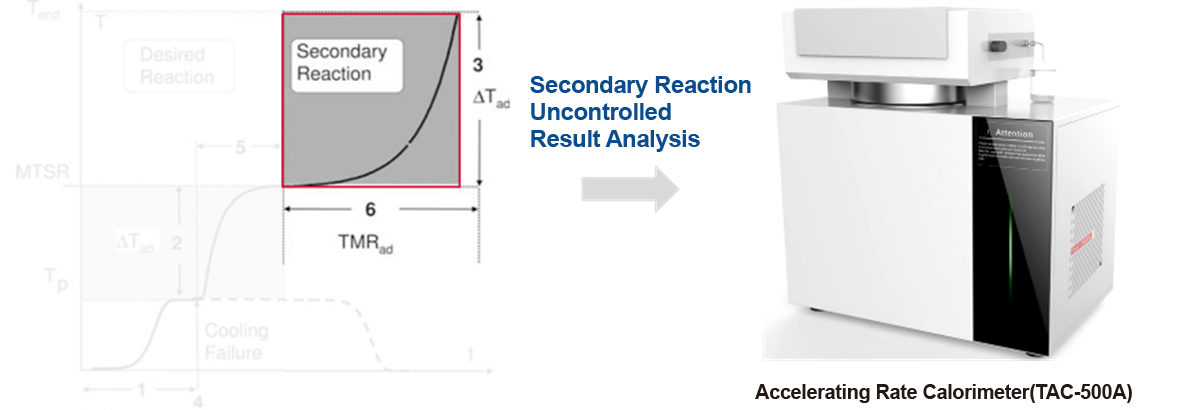
Accelerating Rate Calorimeter
The accelerating rate calorimeter is a professional testing instrument that simulates potential secondary reaction risks under laboratory conditions and can accurately measure the temperature and pressure changes of samples during adiabatic decomposition, especially providing slow pressure changes in the early stage of thermal decomposition that cannot be obtained by techniques like DTA and DSC.
During the testing process of the accelerating rate calorimeter, the sample is placed in the central spherical sample chamber, surrounded by evenly distributed electric heating blocks in the outer cavity. These blocks promptly replenish the heat loss caused by the temperature difference between the sample and its surrounding environment, maintaining the temperature within the adiabatic furnace in a uniform equilibrium state. This setup ensures an ideal adiabatic testing environment within the sample chamber.
The classic operating mode of the accelerating rate calorimeter is the “Heat-Wait-Search” (HWS) mode. Before operation, parameters such as initial temperature, final temperature, slope sensitivity, heating rate, and waiting time can be set. During the “Heat” phase, the temperature of the calorimeter increases according to the set heating rate.
In the “Wait” phase, the controller compares the temperature of the sample chamber with the temperatures in various regions of the adiabatic furnace, ensuring uniform temperature equilibrium within the furnace. In the “Search” phase, if the temperature rise rate of the sample exceeds the set threshold (such as 0.02°C/min), the adiabatic tracking phase is entered, and the adiabatic environment around the sample chamber is maintained through programmed temperature control. Otherwise, the calorimeter automatically proceeds to the next “Heat-Wait- Search” cycle.
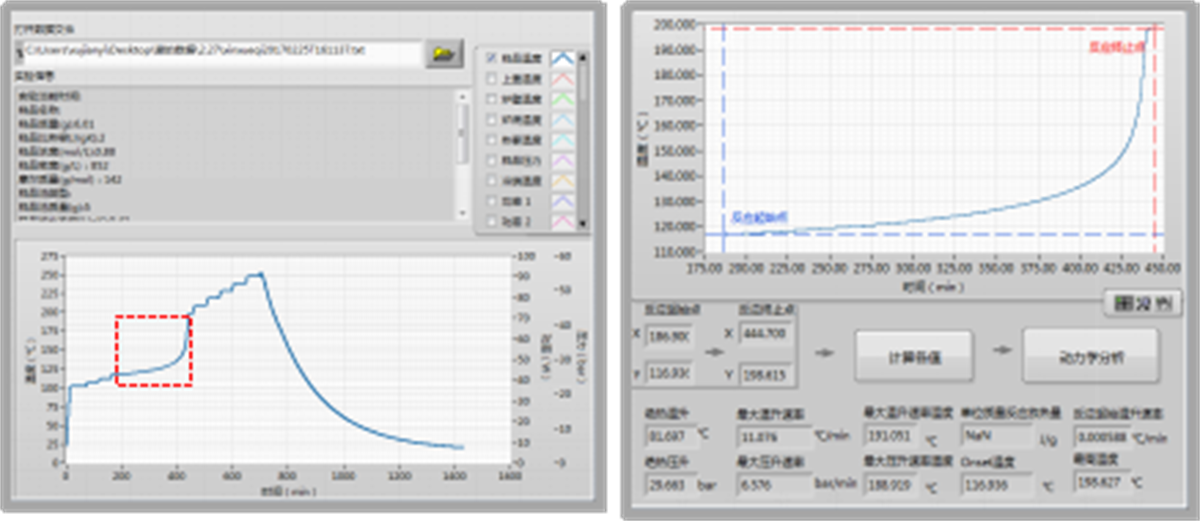
Through the sample temperature rise curve during the adiabatic tracking phase, the adiabatic starting temperature and the adiabatic temperature rise (ΔT ad ) of the decomposition reaction can be determined. By performing kinetic analysis on the temperature rise curve, key parameters for reaction risk assessment such as activation energy (E), pre-exponential factor (A), Time to Maximum Rate of Adiabatic Temperature Rise (TMR ad ), and Time to Decomposition at 24°C (T D24 ) can be further calculated.
The TAC-500A is the first domestically developed accelerating rate calorimeter. This instrument enables rapid temperature tracking, ensuring good measurement accuracy even at lower phi factors. It features a practical desktop compact design, easy maintenance, and a favorable ratio of usage to maintenance time . Currently, this instrument is widely recognized by customers in China and has successfully entered the European market.
Reference
[1] In-depth Industry Insight: Chemical Industry In-Depth: Segment Value Analysis and Track Targets Combing https://www.tamigos.com/news/27955
[2] 2019 National Chemical Accident Analysis Report