Investigating Flash Point Behavior in Flammable Liquid Solutions — Microscale Continuously Closed Cup Flash Point Tester

Introduction – Flash Point Behavior
Flash point, a critical parameter characterizing the volatility and flammability of liquid or solid substances, holds significant importance in various industries. Particularly, in sectors like coatings, pesticides, pharmaceuticals, and fine chemicals, where solutions of flammable liquids and water are commonly used, determining the flash point plays a crucial role in identifying fire and explosion risks, as well as in devising control measures. In this study of Flash Point Behavior, conducted using the FP CC-420A Microscale Continuous Closed Flash Point Tester, the relationship between the flash point and the concentration of three typical flammable liquid-water solutions was investigated. Furthermore, a comparative analysis between the conventional Closed Cup Tester (ASTM D6450) and the improved Continuous Closed Cup Tester (ASTM D7094) methods was performed to understand differences in test results.
 Microscale Continuous Closed Flash Point Tester(FP CC-420A)
Microscale Continuous Closed Flash Point Tester(FP CC-420A)
Sample Preparation
The experiment utilized three substances: ethanol, acrylic acid, and dimethylacetamide, which were mixed with pure water to prepare solutions of varying concentrations.
Table 1 Material information sheet
|
Material Name |
Specification |
Boiling Point(°C) |
Open Flash Point(°C) |
|
Acrylic acid |
CP |
140.9 |
54 |
|
Dimethylacetamide |
CP |
166 |
66 |
|
Anhydrous Ethanol |
AR |
78 |
12 |
Experimental Results
The flash point values of ethanol, acrylic acid, and dimethylacetamide solutions exhibited a monotonic increase with dilution, as depicted in Figure 1. Despite these solutions not adhering strictly to Raoult’s Law, wherein the vapor phase mole fraction of flammable components generally increases with the liquid phase mole fraction, the saturation vapor pressure of flammable liquids in dilute solutions remains relatively low. Consequently, a higher temperature is required to reach the Lower Flammable Limit (LFL) of organic vapors.
Fig. 1 Flash point dilution curves of (a) ethanol, (b) acrylic acid and (c) dimethylacetamide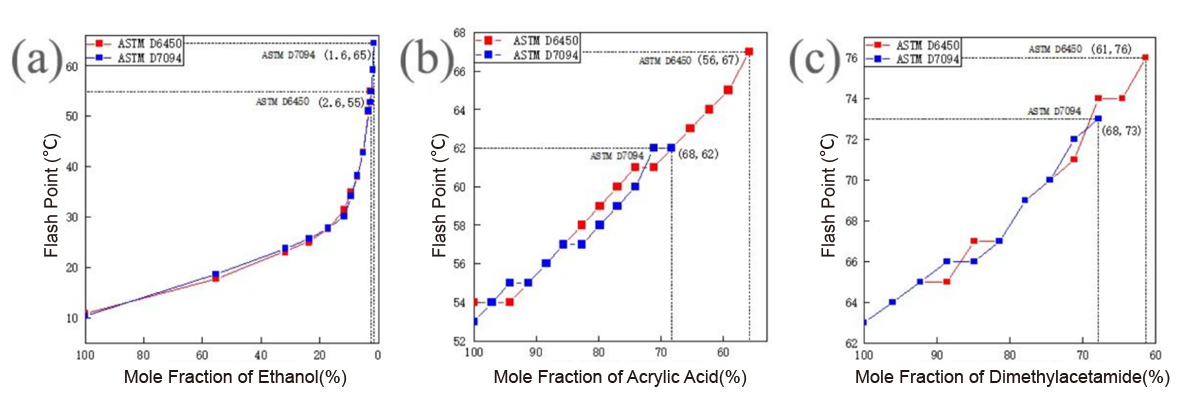
The detection limits of flash points for the three samples were determined to be 1.6% for ethanol, 56% for acrylic acid, and 61% for dimethylacetamide. Below these limits, due to the inert water vapor pressure within the testing chamber being too high, flash ignition of organic vapors cannot occur. Additionally, the detection limit correlates positively with the boiling point and open-cup flash point values of pure substances. Hence, it can be inferred that the stronger the volatility and flammability of the sample, the wider the concentration range within which flash ignition can occur.
Both ASTM D6450 and ASTM D7094 methods demonstrated high consistency in determining flash point values at single concentrations. However, differences in sample size, heating rate, air introduction, etc., led to variations in the concentration detection limits, as illustrated in Figure 2 and Table 1. The accumulation of vapor concentration to the LFL during the testing process is a complex nonlinear, multi-factor coupled process, wherein the concentration limit does not exhibit a clear correlation with the detection method.
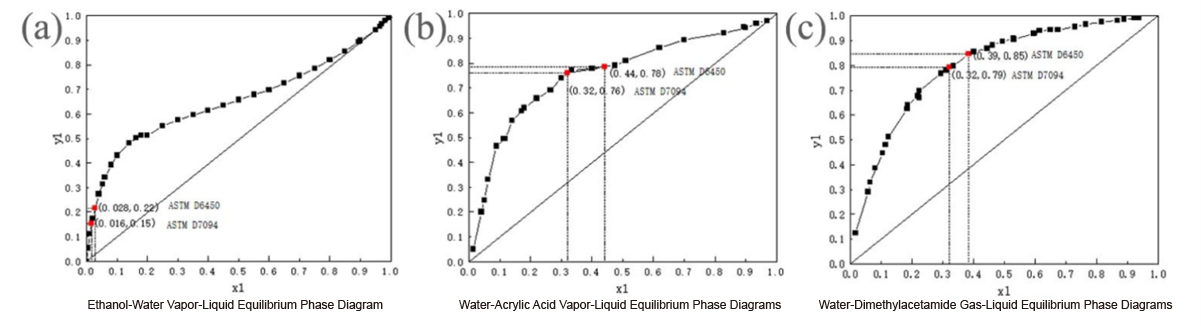
Fig. 2 Gas-liquid equilibrium phase diagrams for the binary systems of (a) ethanol, (b) acrylic acid and (c) dimethylacetamide with water.
|
Sample |
ASTM D7094 |
ASTM D6450 |
||
|
Lower Concentration Limit% |
Organic Vapor Mole Fraction% |
Lower Concentration Limit% |
Organic Vapor Mole Fraction% |
|
|
Anhydrous ethanol |
1.6 |
15 |
2.6 |
22 |
|
Acrylic acid |
68 |
24 |
56 |
22 |
|
Dimethylacetamide |
68 |
21 |
61 |
15 |
Conclusion
The flash point of flammable liquid-water solutions increases monotonically with dilution, while the detectable concentration range depends on the volatility and flammability of pure substances, as well as the testing method employed.






































