Vapor Pressure Apparatus: A Comprehensive Guide
Vapor pressure is a crucial physical property of a liquid that determines its boiling point, evaporation rate, and volatility. It is defined as the pressure exerted by the vapor of a substance in equilibrium with its liquid phase at a given temperature. The vapor pressure of a liquid increases with temperature and depends on the intermolecular forces between its molecules. The measurement of vapor pressure is essential in many industries, including petroleum, chemical, pharmaceutical, and food, to ensure product quality, safety, and compliance with regulations.
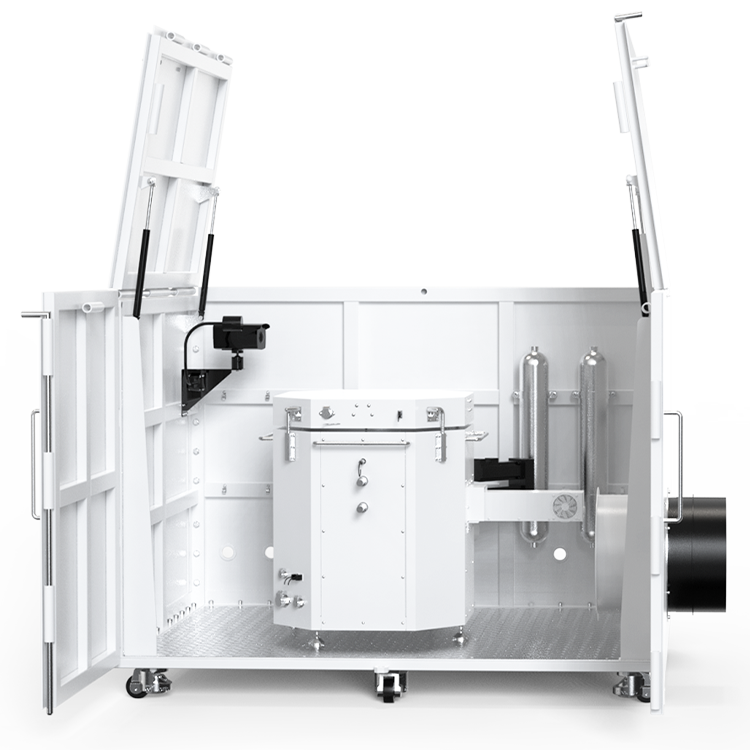
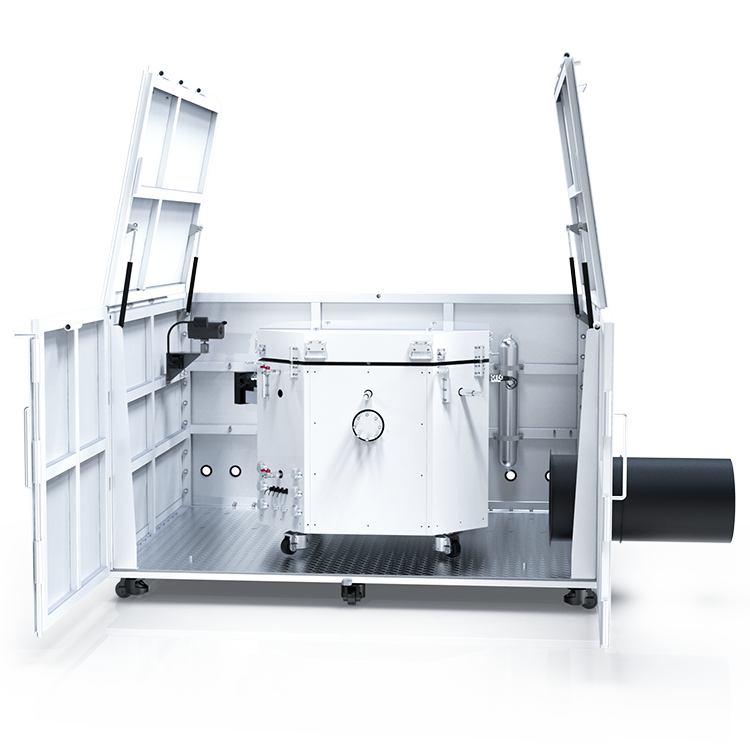
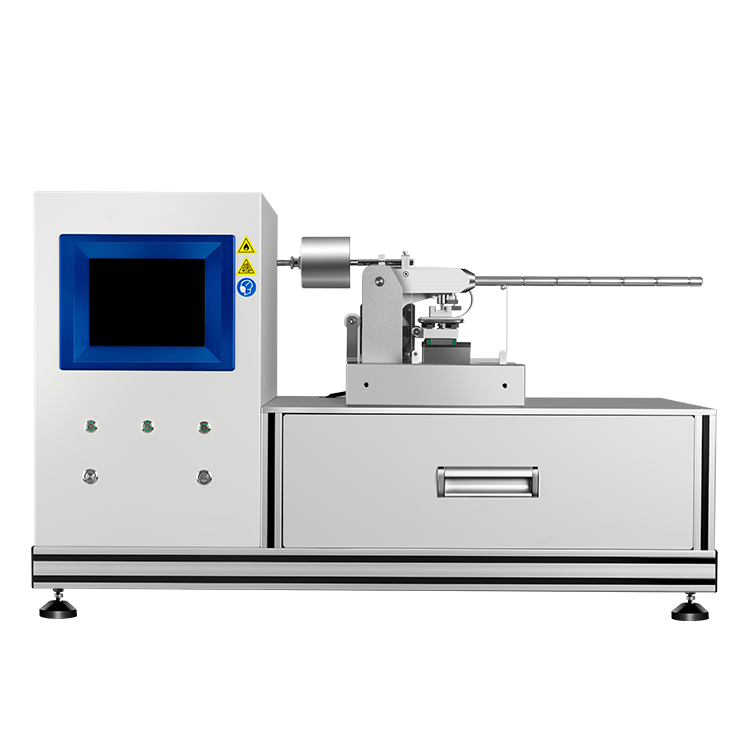
Vapor pressure apparatus is a sophisticated instrument that measures the vapor pressure of liquids accurately and precisely. It consists of several components, such as a sample cell, a temperature control system, a pressure transducer, and a data acquisition system. The operating procedures of vapor pressure apparatus involve filling the sample cell with a known volume of liquid, equilibrating it at a specific temperature, and measuring the pressure of the vapor phase. The types of vapor pressure apparatus vary depending on the temperature range, pressure range, and sample size, ranging from manual methods to automated systems.
Key Takeaways
- Vapor pressure is a critical parameter that affects the physical and chemical properties of liquids and is measured by vapor pressure apparatus.
- Vapor pressure apparatus consists of various components and operates by filling a sample cell with liquid, equilibrating it, and measuring the vapor pressure.
- The types of vapor pressure apparatus vary depending on the temperature range, pressure range, and sample size, and are used in many industries for quality control, safety, and regulatory compliance.
Fundamentals of Vapor Pressure
Definition and Concepts
Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The vapor pressure of a liquid increases with temperature, as the temperature increases, the vapor pressure also increases. Conversely, the vapor pressure decreases as the temperature decreases.
The concept of vapor pressure is important in understanding various phenomena such as boiling, evaporation, and condensation. For example, when a liquid is heated, its vapor pressure increases and eventually reaches atmospheric pressure, causing the liquid to boil. Similarly, when a liquid evaporates, its vapor pressure increases until it reaches a point of equilibrium with the surrounding atmosphere.
Measurement Principles
The vapor pressure of a liquid can be measured using a vapor pressure apparatus. The apparatus consists of a sample chamber, a temperature control system, and a pressure measurement system. The sample chamber is filled with the liquid whose vapor pressure is to be measured. The temperature of the sample chamber is controlled by a temperature control system, and the pressure of the vapor above the liquid is measured by a pressure measurement system.
There are several methods for measuring vapor pressure, including the static method, the dynamic method, and the isoteniscope method. In the static method, the vapor pressure of the liquid is measured at a constant temperature. In the dynamic method, the vapor pressure is measured as a function of temperature. The isoteniscope method involves measuring the vapor pressure of a liquid at different temperatures while keeping the liquid volume constant.
Overall, the measurement of vapor pressure is an important tool for understanding the thermodynamic properties of liquids and their behavior under different conditions.
Vapor Pressure Apparatus Components
If you are planning to measure the vapor pressure of a liquid, you will need a vapor pressure apparatus. This apparatus consists of several components that work together to provide accurate and reliable measurements. In this section, we will discuss the three main components of a vapor pressure apparatus: the sample container, pressure sensor, and temperature control system.
Sample Container
The sample container is where the liquid to be measured is placed. It is typically made of glass or metal and is designed to withstand high temperatures and pressures. The container is sealed to prevent any air or moisture from entering, which could affect the accuracy of the measurement. The volume of the sample container is also important, as it affects the accuracy of the measurement. The container should be large enough to hold the liquid sample but not so large that it affects the vapor pressure.
Pressure Sensor
The pressure sensor is a critical component of the vapor pressure apparatus. It measures the pressure of the vapor above the liquid sample. The pressure sensor is typically a transducer that converts the pressure into an electrical signal that can be read by a computer or other device. The accuracy of the pressure sensor is important for obtaining accurate measurements. The pressure sensor should be able to measure pressures in the range of a few millimeters of mercury to several atmospheres.
Temperature Control System
The temperature control system is used to maintain a constant temperature during the measurement. The temperature of the liquid sample affects the vapor pressure, so it is important to control the temperature to obtain accurate measurements. The temperature control system typically consists of a water bath or heating mantle, a temperature sensor, and a controller. The temperature sensor measures the temperature of the liquid sample, and the controller adjusts the temperature of the water bath or heating mantle to maintain the desired temperature.
In summary, the vapor pressure apparatus consists of three main components: the sample container, pressure sensor, and temperature control system. These components work together to provide accurate and reliable measurements of the vapor pressure of a liquid. The accuracy of the measurement depends on the quality of these components and how well they are calibrated and maintained.
Operating Procedures
When using a vapor pressure apparatus, it is important to follow proper operating procedures to ensure accurate and reliable results. This section covers the sample preparation, calibration and standardization, and data acquisition procedures that you should follow when using a vapor pressure apparatus.
Sample Preparation
Before conducting any measurements, you must prepare your sample according to the appropriate laboratory procedure. This may include filtering, diluting, or otherwise manipulating the sample to ensure that it is in the proper state for measurement. Follow the laboratory procedure carefully to ensure that your sample is prepared correctly.
Calibration and Standardization
Before taking any measurements, you must calibrate and standardize your apparatus. This involves setting the apparatus to known standards and checking that it is reading accurately. Follow the manufacturer’s instructions for calibration and standardization, and ensure that you are using the appropriate standards for your specific apparatus.
Data Acquisition
When acquiring data, follow the manufacturer’s instructions for your specific apparatus. This may involve taking multiple measurements and averaging the results, or taking measurements at specific intervals. Record your results carefully, including any relevant information such as the date, time, and environmental conditions.
In addition to these procedures, it is important to follow all relevant laboratory safety procedures. Wear appropriate personal protective equipment, work in a well-ventilated area, and follow all relevant safety protocols. By following these procedures, you can ensure that your measurements are accurate and reliable, and that you are working safely and responsibly in the laboratory.

Applications and Uses
Vapor pressure apparatus is a versatile tool that finds applications in various industries, research and development, and quality control. Here are some of the uses of vapor pressure apparatus:
Industrial Relevance
In industries such as petrochemical, pharmaceutical, and food processing, vapor pressure apparatus is used to measure the vapor pressure of liquids. The measurement of vapor pressure is crucial in the production and storage of these products. For instance, in the petrochemical industry, vapor pressure measurements are used to determine the volatility of crude oil, which is essential in the production of gasoline and other fuels.
Research and Development
In the research and development of new products, vapor pressure apparatus is used to determine the vapor pressure of new compounds. The vapor pressure of a compound is an important physical property that affects its behavior in various applications. For example, in the development of new refrigerants, vapor pressure measurements are used to determine the boiling point and the efficiency of the refrigerant.
Quality Control
Vapor pressure apparatus is also used in quality control to ensure the consistency and quality of products. In the food industry, vapor pressure measurements are used to determine the quality and freshness of food products. For instance, the freshness of coffee beans can be determined by measuring the vapor pressure of the volatile compounds released by the beans.
In summary, vapor pressure apparatus is a valuable tool in various industries, research and development, and quality control. Its ability to measure the vapor pressure of liquids makes it an essential tool in the production, storage, and development of new products.